$\mathop {C{H_3} - \mathop C\limits^ \oplus H - C{H_3}}\limits_I $
$\mathop {C{H_3} - \mathop C\limits^ \oplus H - OC{H_3}}\limits_{II} $
$\mathop {C{H_3} - \mathop C\limits^ \oplus H - C{H_2} - OC{H_3}}\limits_{III} $
Stability of the given cations can be understood by the following structures
\(\mathop {\mathop {C{H_3} \to \mathop C\limits^ \oplus H \leftarrow C{H_3}}\limits_I }\limits_{ + I - effect\,of\,two\,methyl\,group\,stabilise\,the\,carbocation\,(I)} \) ;
\(\mathop {\mathop {C{H_3} \to \mathop C\limits^ \oplus H - \mathop O\limits_{\,}^{\,} - C{H_3}}\limits_I }\limits_{Strong\, + R - effect\,of\, - OC{H_3}\,group\,stabilise\,the\,carbocation\,(II)} \)
\(\mathop {\mathop {C{H_3} - \mathop C\limits^ \oplus H - C{H_2} \to OC{H_3}}\limits_{III} }\limits_{ - I - effect\,of\, - OC{H_{3\,}}group\,stabilise\,the\,carbocation\,(III)} \)
Thus, the stability of carbocation decreases in the order,
\(I I>I>I I I\)
Download our appand get started for free
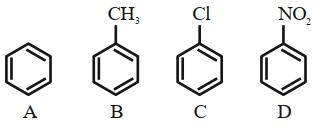
Similar Questions
- 1View Solutionકોના કારણે સંસ્પદન થાય છે ?
- 2View Solutionનીચેના પદાર્થો માટે ઈલેકટ્રો અનુરાગી વિસ્થાપન પ્રક્રિયા માટે પ્રક્રિયા શીલતાનો સાચો ક્રમ કયો છે ?
- 3View Solutionએસિડ્સની કેટલીક જોડીઓ નીચે આપેઌ છે. તે જોડી પસંદ કરો જેમાં પ્રથમ એસિડ વધુ પ્રબળ હોય
- 4View Solutionનીચે પૈકી જે કેન્દ્રાનુરાગી અને ઇલેક્ટ્રોનુરાગી બંને તરીકે વર્તે છે?
- 5View Solutionતેમની વ્યક્તિગત સ્થિરતા માટે ફોર્મિક એસિડની નીચેની ગુંજારિત રચનાઓની તપાસ કરો અને પછી નીચે આપેલા સવાલનો જવાબ આપો.
નીચે આપેલમાંથી કઈ ગોઠવણી ઉપરોક્ત સંસ્પંદન ફાળો આપનારાઓની સ્થિરતામાં ઘટાડો કરવાનો યોગ્ય ક્રમ આપે છે?
- 6એસિડિક પ્રબળતાના વધતા ક્રમમાં નીચેનાને ફરીથી ગોઠવો.View Solution
$(i)$ બેઞ્ઝોઈક એસિડ $(ii)\, p$ - મિથોક્સિબેઞ્ઝોઈક એસીડ $(iii)\, o$ - મિથોક્સિબેઞ્ઝોઈક એસિડ
- 7View Solutionસંકરણ અને સંસ્પંદન અસરને ધ્યાનમાં લેતા, બંધ લંબાઈ ઘટતી ક્રમમાં નીચેના બંધને ક્રમ આપો.
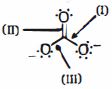
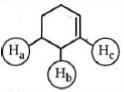
- 8એસિડિક પ્રબળતા અનુસાર નીચેના પરમાણુઓમાં હાઇડ્રોજન અણુઓ $(H_a , H_b, H_c,)$ ને ક્રમ આપોView Solution
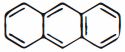
- 9View Solutionએન્થ્રેસીન માટે કેટલા સંસ્પંદન બંધારણ છે?
- 10View Solutionઈલેકટ્રોન અનુરાગી વિસ્થાપન પ્રક્રિયા પ્રત્યેની સક્રિયતાનો ક્રમ જણાવો.