નીચેના કયા પરિમાણો માટે બંધારણીય સમઘટકતા ${C_2}{H_5}OH$ અને $C{H_3}OC{H_3}$ સમાન મૂલ્યોની અપેક્ષા રાખવામાં આવશે? (આદર્શ વર્તણૂક ધારો)
AIEEE 2004, Medium
d
(d) Gaseous density of both ethanol and dimethyl ether would be same under identical condition of temperature and pressure while the rest of these three properties vapour pressure, boiling point and heat of vaporization will differ as ethanol has hydrogen bonding where as ether does not.
(d) Gaseous density of both ethanol and dimethyl ether would be same under identical condition of temperature and pressure while the rest of these three properties vapour pressure, boiling point and heat of vaporization will differ as ethanol has hydrogen bonding where as ether does not.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionઆપેલા પરમાણુઓમાંથી કયું ચલરૂપકતા પ્રદર્શિત કરી શકે છે?
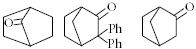
- 2View Solutionકયા અણુમાં બે કીરાલ કાર્બન પરમાણુઓ આવેલા નથી ?
- 3View Solutionનીચેનામાંથી ક્યો પદાર્થ પ્રકાશ સમઘટકતા દર્શાવશે ?
- 4View Solutionકયો પદાર્થ ચલરૂપકતા સ્વરૂપે અસ્તિત્વ ધરાવે છે ?
- 5$2, 3$ -ડિક્લોરો બ્યુટેન માટે શક્ય અવકાશરસાયણ ની કુલ સંખ્યા કેટલી છે ?View Solution
- 6$3-$ ઇથાઇલ $-2-$ મિથાઇલ હેકઝેનનો સમઘટક હોય તેવો સળંગ શૃંખલા ધરાવતો હાઇડ્રોકાર્બન જણાવો.View Solution
- 7$2 -$મિથાઈલ બ્યુટેનના મોનોક્લોરીનેશન કરતાં મળતાં પદાર્થોમાં કેટલા કિરાલ પદાર્થો મળે છે ?View Solution
- 8View Solutionકયા પદાર્થ માટે ભૌમિતિક સમઘટકો શક્ય છે ?
- 9સૂચિ $I$ સાથે સૂચિ $II$ ને જોડો.View Solution
સૂચિ $I$ સૂચિ $II$ $A$ પ્રોપેનામાઈન અને $N-$મિથાઈલ ઈથેનામાઈન $I$ મધ્યાવયવી $B$ હેકઝેન$-2-$ઓન અને હેક્ઝેન$-3-$આોન $II$ સ્થાન સમઘટકો $C$ ઈથેનામાઈડ અને હાઈડ્રોકસીઈથેનામાઈન $III$ ક્રિયાશીલ સમઘટકો $D$ $o-$નાઈટ્રોફિનોલ અને $p-$નાઈટ્રોફિનોલ $IV$ ચલરૂપકો નીચે આપેલ વિકલ્પોમાંથી સાચો જવાબ પસંદ કરો.
- 10View Solutionબતાવેલ બે બંધારણો વચ્ચે શું સંબંધ છે?