$(i)$ $\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{C{H_3} - {C^ \mathbf{-} }} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{H_3}}
\end{array}$
$(ii)\;\;CH_2 = CH -\stackrel{\mathbf{-}}{C}H_2$
$(iii)\;\;CH \equiv \stackrel{\mathbf{-}}{C}$
$(iv)\;\;\stackrel{\mathbf{-}}{C}H_3$
$(v)\;\;\stackrel{\mathbf{-}}{C}N$
Basicity \(\propto \frac{1}{\text { Stability }}\)
\((v)\) In figure \(5 , sp\) hybridised carbon is more electronegative. -ve charge on more electronegative atom makes it more stable. [ref. image]
Also, there is \(-I\) effect.
\(\therefore\) It is most stable.
\((iii)\) \(H - C \equiv \overline{ C }\)
sp hybridized carbon carry -ve charge.
\(\therefore\) Hence, it is also more stable
\((ii)\) In figure \(2\), resonance is taking place. [ref. image]
\(\therefore\) It is more stable.
\((iv)\) \(\overline{ C } H _3\)
-ve charge is carried by \(sp ^3\) hybridised carbon.
\(\therefore\) It is less stable.
\((i)\) In figure \(1\) , the central \(C\) is surrounded by \(CH _3\) and hence it shows \(+ I\) effect from \(3\) sides. [ref. image]
\(\therefore\) Hence, it is least stable.
\(\therefore \text { (v) }\,<\,\text { (iii) }\,<\,\text { (ii) }\,<\,\text { (iv) }\,<\,\text { (i) }\)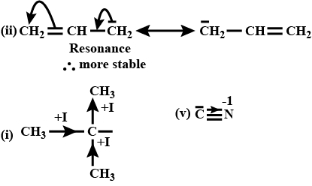
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાંથી કયો જલીય માધ્યમમાં પ્રબળ બેઈઝ છે ?
- 2View Solutionઝાયલેન્સમાં, જે ઉષ્માગતિકીયરીતે સૌથી સ્થાયી કયું છે?
- 3View Solutionનીચે આપેલા માટે સૌથી વધુ સ્થાયી કાર્બોકેશાયન શોધી.
- 4$\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CHO}$ ના નીચે આપેલા સસ્પંદન બંધારણો નો સાચો સ્થિરતા ક્રમ શાધોView Solution
- 5કાર્બોકેટાયનને તેમની ઘટતી સ્થિરતાના ક્રમમાં ગોઠવો.View Solution
$(1) H_3C - C = C⊝\,(2) H - C = C⊝ \,(3) $ ${H_3}C\,\, - \,\,\mathop C\limits^\Theta {H_2}$
- 6View Solutionનીચેનામાંથી કયા સ્થિર કાર્બોકેટાયન છે?
- 7View Solutionઘટતા ક્રમમાં નીચેનામાંથી કોનું સ્થળાંતર યોગ્ય છે ?
- 8એસિડ સાથેની પ્રક્રિયા પર, $4$ -પાયરોન ખૂબ જ સ્થિર ધનાયન નીપજ આપે છે.નીચેનામાંથી કયું બંધારણ પ્રોટોનેશન બાજુને તે નીપજ બતાવે છે?View Solution
- 9View Solutionનીચેનામાંથી કોણ સૌથી ઓછો એસિડિક છે ?
- 10View Solutionનીચેના પદાર્થોમાં કેન્દ્રાનુરાગી વિસ્થાપન માટે પ્રક્રિયાશીલતાનો ઘટતો ક્રમ કયો છે ?