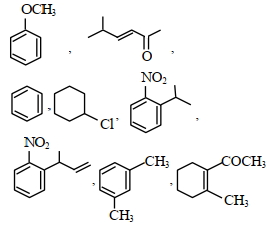
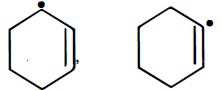
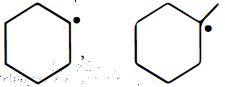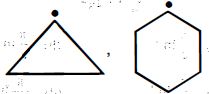નીચેના પૈકી કઇ જોડમાં $B$ કરતા $A$ વધુ સ્થાયી છે ?
$A$ || $B$
JEE MAIN 2014, Diffcult
d
\(P{{h}_{3}}\overset{\centerdot }{\mathop{C}}\,\) is more stable than \({{\left( C{{H}_{3}} \right)}_{3}}\overset{\,\,\centerdot }{\mathop{C}}\,\) because resonance stabilisation effect in \(P{{h}_{3}}\overset{\centerdot }{\mathop{C}}\,\) is more pronounced as compared to hyperconjugation stabilisation effect in \({{\left( C{{H}_{3}} \right)}_{3}}\overset{\,\,\centerdot }{\mathop{C}}\,\) overall stability order among free radical is : Triphenylmethyl \(>\) benzyl \( >\) allyl \(>\) tertiary alkyl \(>\) secondary \(>\) primary \(>\) methyl \(>\) vinyl
\(P{{h}_{3}}\overset{\centerdot }{\mathop{C}}\,\) is more stable than \({{\left( C{{H}_{3}} \right)}_{3}}\overset{\,\,\centerdot }{\mathop{C}}\,\) because resonance stabilisation effect in \(P{{h}_{3}}\overset{\centerdot }{\mathop{C}}\,\) is more pronounced as compared to hyperconjugation stabilisation effect in \({{\left( C{{H}_{3}} \right)}_{3}}\overset{\,\,\centerdot }{\mathop{C}}\,\) overall stability order among free radical is : Triphenylmethyl \(>\) benzyl \( >\) allyl \(>\) tertiary alkyl \(>\) secondary \(>\) primary \(>\) methyl \(>\) vinyl
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1ઈલેકેટ્રોન અનુરાગી $ E^{\oplus} $ બેન્ઝિન ચક્ર પર હુમલો કરીને મધ્યવર્તીં $ \sigma -$ સંકીર્ણ બનાવે છે. તેમાંથી કયો $\sigma - $ સંકીર્ણ સૌથી ઓછી ઉર્જા ધરાવે છે ?View Solution
- 2View Solutionઉષ્મા દહન માં ઘટાડો થતાં ક્રમમાં નીચેના પદાર્થોને નંબર આપો (મહતમ થી ન્યૂનતમ )

- 3View Solutionનીચે આપેલા માટે સૌથી વધુ સ્થાયી કાર્બોકેશાયન શોધી.
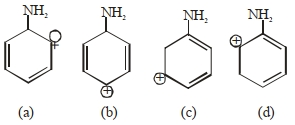
- 4View Solutionકયો એસિડ સૌથી પ્રબળ છે ?
- 5View Solutionઆપેલ અણુની સૌથી સ્થાયી પ્રમાણભૂત રચના કઈ છે:
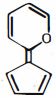
- 6View Solutionઆપેલ બંધારણ વચ્ચે સૌથી સ્થાયી પ્રમાણભૂત બંધારણ કયું છે
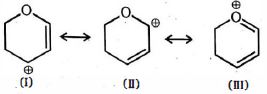
- 7પીક્રીક એસિડ, એસિટિક એસિડ અને ફિનોલના $ H_2O$ માં $ pK_a $ મૂલ્ય કયા ક્રમમાં છેView Solution
- 8View Solutionનીચેનામાંથી કયુમાંથી કોઈ ડાઈન તમે સૌથી વધુ સ્થાયી રહેવાની અપેક્ષા રાખશો?
- 9આલ્કાઇલ સમૂહો .......... ને કારણે $o-$ તથા $p-$ નિર્દેશક છે.View Solution
- 10View Solutionનીચેના સંયુક્તો માંથી કેટલાક સંયુક્તો ઇન્ડક્ટિવ, મેઝોમેરીક અને હાયપરકન્જગેશન પ્રભાવો બતાવે છે?