નીચેના પૈકી ક્યા આયનની ચુંબકીય ચાકમાત્રા $2.83\, BM$ થશે ?
(પરમાણ્વીય ક્રમાંક : $Ti = 22,\,Cr = 24,$ $ Mn = 25,\,Ni = 28$)
NEET 2014, Medium
b
Magnetic moment is given by \(\mu=\sqrt{n(n+2)}\)
Magnetic moment is given by \(\mu=\sqrt{n(n+2)}\)
Here, \(n=\) number of unpaired electrons
\(\Rightarrow 2.83=\sqrt{n(n+2)}\)
\(\Rightarrow(2.83)^{2}=n(n+2)\)
\(0=8.00+n^{2}+2 n\)
\(n^{2}+ 2n-8=0\)
\((n+4)(n-2)=0\)
\(n=2\) \(n=-\,4\)
\(-\,4\) not be consider
Hence, \(Ni^{2+}\) possesses a magnetic moment of \(2.83\,\mathrm{B} . \mathrm{M}\)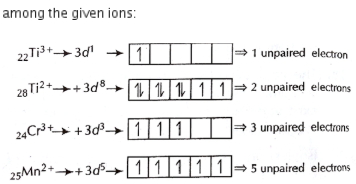
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેની કઈ જોડીઓમાંથી બંને જલીય દ્રાવણમાં રંગીન આયન છેView Solution
(આણ્વિય નંબર . : $Sc = 21, Ti = 22, Ni = 28,$$ Cu = 29, Co = 27$)
- 2View Solutionક્રોમાઈટ ખનીજમાં આયર્ન અને ક્રોમિયમનો ઓક્સિડેશન આંક અનુક્રમે કેટલો હશે ?
- 3View Solutionઆધુનિક આવર્ત કોષ્ટકમાં ક્યા સમૂહના તત્વોને d- વિભાગના તત્વો કહે છે
- 4View Solutionનરમ સ્ટીલમાં કાર્બનનું ટકાવાર પ્રમાણ કેટલું હોય છે?
- 5View Solutionનીચેનામાંથી કયો આર્યન સૌથી સ્થિર સંકીર્ણ સંયોજન બનાવે છે ?
- 6એસિડિક $KMnO_4$ ને ઝડપથી રંગવિહિન કરતા વાયુનું નામ આપો.View Solution
- 7View Solutionનીચેના પૈકી કયા ધાતુ આયનનો રંગ જાબુંડીયો છે
- 8$[Ni (H_2O)_6]^{2+ } $ ના જલીય દ્રાવણનો રંગ કેવો હોય છેView Solution
- 9View Solutionનીચેનામાંથી કયો પદાર્થ તત્વ નથી ?
- 10View Solutionસૌથી વધુ તૃતીય આયનીકરણ એન્થાલ્પી ધરાવતી સંક્રાંતિ ધાતુ શોધો.
