નીચેના પૈકી ક્યો ધટક સમતલીય ત્રિકોણ આકાર ધરાવે છે ?
NEET 2014, Medium
b
Species with $s p^{2}$ hybridisation are plane triangular in shape. Among the given species $N O_{3}^{-}$ is $s p^{2}$ hybridised with no lone pair of electrons on central atom, $N$. Whereas, $N_{3}, N O_{2}^{-}$ and $CO_{2}$ are sp hybridised with a linear shape.
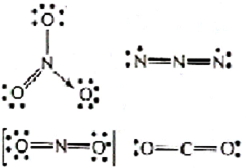
Species with $s p^{2}$ hybridisation are plane triangular in shape. Among the given species $N O_{3}^{-}$ is $s p^{2}$ hybridised with no lone pair of electrons on central atom, $N$. Whereas, $N_{3}, N O_{2}^{-}$ and $CO_{2}$ are sp hybridised with a linear shape.

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેના માંથી ક્યો બંધ મજબુત છે
- 2$M$ ધાતુની ઇલેક્ટ્રોન રચના $1s^2 \,2s^2\,2p^6\,3s^1$ છે, તો તેના ઓક્સાઇડનું સૂત્ર શુ થશે ?View Solution
- 3View Solutionનીચેનામાંથી ક્યા સેટમાં હાઇડ્રોજન બંધન સૌથી પ્રબળ છે?
- 4$C - H, C - O, C - C$ અને $C=C$ બંધલંબાઇનો સાચો વધતો ક્રમ ક્યો છે ?View Solution
- 5${N_2}$અને ${O_2}$ અનુક્રમે $N_2^ + $ અને $O_2^ + $ મોનો કેટાયનમાં ફેરવાય છે તે માટે નીચેનામાંથી શું ખોટું છે?View Solution
- 6$MOT$ મુજબ, ${O}_{2}^{2-}$માં અયુગ્મિત ઇલેક્ટ્રોન(ઓ)ની સંખ્યા $......$ છે.View Solution
- 7નીચેની માહિતી ધ્યાનમાં લો: $(X = F$ અથવા $Cl)$View Solution
અણુ $P-X$ (અક્ષીય) બંધ લંબાઇ $P-X$ (વિષુવવૃતીય) બંધ લંબાઇ $PF_5$ $a$ $b$ $PF_4CH_3$ $c$ $d$ $PF_3 (CH_3)_2$ $e$ $f$ $PCl_5$ $g$ $h$ આપેલ માહિતી અનુસાર બંધલંબાઈનો ખોટો ક્રમ પસંદ કરો
- 8$CS_2$ માટે સૌથી વધારે સ્થાયી લુઇસ બંધારણ વિશે કયુ વિધાન સાચું છે?View Solution
- 9View Solutionજે ઘટકમાં કેન્દ્રીય અણુ તેના જોડાણમાં સંકર ભ્રમણકક્ષાનો ઉપયોગ કરે છે તે કયું છે ?
- 10ટેટ્રાસાયનો ઇથિલીનમાં $\sigma-$ બંધ અને $\pi-$ બંધ વચ્ચેનો ગુણોતર કેટલો છે?View Solution
