નીચેના પૈકી ક્યૂ વિધાન ખોટું છે $?$
JEE MAIN 2013, Medium
$(a)$ Number of electrons in$\begin{array}{l}\mathrm{ONCl}=3.2 \mathrm{e}^{-} \\\mathrm{ONO}^{-}=24 \mathrm{e}^{-}\end{array}$
Thus, $\ce{ONCl}$ and $\ce{ONO}-$ are not isoelectronic.
$(b)\ \mathrm{O}_{3}$ has bent shape structure. Central atom $O$ is $s p^{2}$ hybridised with $1$ lone pair of electrons.
$(c)$ Ozone is violet$-$black in solid state.
$(d)\ O_{3}$ has no unpaired electrons, thus it is diamagnetic.
Thus, $\ce{ONCl}$ and $\ce{ONO}-$ are not isoelectronic.
$(b)\ \mathrm{O}_{3}$ has bent shape structure. Central atom $O$ is $s p^{2}$ hybridised with $1$ lone pair of electrons.
$(c)$ Ozone is violet$-$black in solid state.
$(d)\ O_{3}$ has no unpaired electrons, thus it is diamagnetic.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
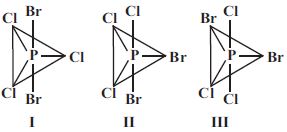
- 1$PCl_3Br_2$ ભૌમિતિક સમઘટકતા દર્શાવે છે, જેના ભૌમિતિક સમઘટકો નીચે મુજબ છે. તે પૈકી કોણ દ્વિધ્રુવ ચાકમાત્રા ધરાવતો(તા) નથી ?View Solution
- 2$I _{3}^{-}$ આયનનો સાચો આકાર અને $I-I-I$ બંધ ખૂણાઓ અનુક્રમે શોધો :View Solution
- 3$P_4O_{10}$ માં સિગ્મા બંધની સંખ્યા .......... છે.View Solution
- 4View Solutionનીચે પૈકી કયું સહસંયોજક સંયોજન છે?
- 5View Solutionનીચેનામાંથી ક્યા સેટમાં હાઇડ્રોજન બંધન સૌથી પ્રબળ છે?
- 6View Solutionપાણી એ પ્રવાહી છે કારણ કે
- 7નીચેના પરમાણુઓ/આયનોના સંકર કક્ષામાં $s-$ગુણધર્મ (ટકાવારીમાં) વધવાનો સાચો ક્રમ કયો છે?View Solution
$(I)\, CO^{2-}_3$ $(II)\, XeF_4$ $(III)\, I^-_3$ $(IV)\, NCl_3$ $(V)$ $BeCl_2$
- 8વિધાન : સિગ્મા $(\sigma )$ પ્રબળ બંધ છે , જ્યારે પાઇ $(\pi )$ એ નિર્બળ બંધ છેView Solution
કારણ : અણુઓ $(\pi )$ બંધ વિશે મુક્તપણે ફરે છે. - 9$A :$ ટેટ્રાસાયનોમિથેનView Solution
$C :$ બેન્ઝિન
$B :$ કાર્બન ડાયોક્સાઇડ
$D : 1, 3-$ બ્યુટા-ડાઇ-ઇન
$\sigma$ અને $\pi$ બંધનો ગુણોતર ક્રમમાં જણાવો - 10$NH_3$ નું ઉત્કલન બિંદુ $PH_3$ ની તુલના માં ઊંચું છે કારણ કેView Solution
