નીચેના સંયોજનો પૈકી એક, જે કેન્દ્રાનુરાગી નાઇટ્રેશન તરફ સૌથી વધુ ક્રિયાશીલ છે તે છે...
AIPMT 1992,AIPMT 2012, Medium
c
The presence of electron releasing group like\(-R.-OH\) etc, increases the electron density at o/p position and thus, makes the benzene ring more reactive (at o/p position) towards electrophile. on the other hand, electron withdrawing group like \(-COOH,-NO_{2} \text { etc. If present, }\)
The presence of electron releasing group like\(-R.-OH\) etc, increases the electron density at o/p position and thus, makes the benzene ring more reactive (at o/p position) towards electrophile. on the other hand, electron withdrawing group like \(-COOH,-NO_{2} \text { etc. If present, }\)
reduces electron density and thus, reduces the activity benzene nucleus towards electrophile. Thus, the order of the given compounds towards electrophilic nitration is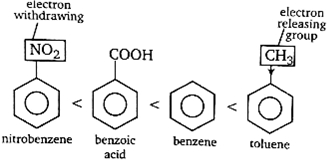
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$HBr$ ના બે અણુઓ $CH_3 - C$View Solution
$CH$ ઉમેરતાં શું મળે છે ?
- 2View Solutionઇથેનનો આકાર........ છે.
- 3ઈથાઈલ બેન્ઝિનનું $KMnO_4$ દ્વારા ઓક્સિડેશન કરતા કયો પદાર્થ મળે છે ?View Solution
- 4View Solutionસિસ સને ટ્રાન્સ- સમઘટ્કતા આપેલ ઉદીપકીય હાયડ્રોજીનેશન પર નીચેનામાંથી ક્યા આલ્કીન આપે છે?
- 5View Solutionટોલ્યુઇનના સંપૂર્ણ નાઇટ્રેશનથી મળતી નીપજ......... છે.
- 6ટોલ્યુઇનની એસિડિક $KMnO_4$ સાથેની પ્રક્રિયાથી ..........મળે છેView Solution
- 7જ્યારે $2$ -બ્યુટાઈન એ $Pd - BaSO_4$ સાથે પ્રકિયા કરે છે ત્યારે નીપજ કઈ મળે છે ?View Solution
- 8$HBr$ સાથેની પ્રક્રિયા શું આપે છે?View Solution
- 9View Solutionબતાવેલ તમામ હાઇડ્રોકાર્બન ખૂબ નબળા એસિડ્સ છે. એક જોકે અન્ય કરતા વધુ એસિડિક છે. કયું એક સૌથી મજબૂત એસિડ છે
- 10$C _{4} H _{8}$ અણુસૂત્ર સાથે ના બે સમધટકો $'A'$ અને $'B'$ની એસિડિક માધ્યમમાં $KMnO _{4}$ સાથે રિડક્શન પ્રક્રિયા કરતા જુદ્દી જુદી નીપજો પ્રાપ્ત થાય છે. સમઘટક $'A'$ ની $KMnO _{4} / H ^{+}$સાથે પ્રક્રિયા કરતાં પરિણામ સ્વરૂપે એક વાયુના ઉભરા અને એક કિટોન મળે છે. તો સંયોજન $'A'$ શોધો.View Solution
