નીચેના સંયોજનો પૈકી કયું સંયોજન એવું છે કે જે ધ્રુવીય છે અને તેમાં કેન્દ્રિય અણુનું સંકરણ $s{p^2} - $ છે
IIT 1997, Medium
a
(a) In \({H_2}C{O_3}\) and \(B{F_3}\) central atom are in \(s{p^2}\) hybridization but in \({H_2}C{O_3}\) due to the ionic character of \(O - H\) bond it will be polar (High electronegativity of oxygen).
(a) In \({H_2}C{O_3}\) and \(B{F_3}\) central atom are in \(s{p^2}\) hybridization but in \({H_2}C{O_3}\) due to the ionic character of \(O - H\) bond it will be polar (High electronegativity of oxygen).
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી કયા બંધમાં સૌથી ઓછી બંધ ઊર્જા છે?
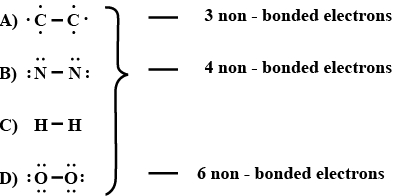
- 2$P_4O_{10}$ માં સિગ્મા બંધની સંખ્યા .......... છે.View Solution
- 3નીચેનામાંથી કયા પરમાણુઓ આંતર-આણ્વિય $H-$બંધનનું પ્રદર્શન કરે તેવી અપેક્ષા છે?View Solution
$(I)$ એસિટિક એસિડ $(II)\, o-$ નાઇટ્રોફિનોલ $(III)\, m-$ નાઇટ્રોફિનોલ $(IV)\, o-$ બોરિક એસિડ
સાચો વૈકલ્પિક પસંદ કરો
- 4$MO$ સિદ્ધાંત અનુસાર યાદીમાંના નાઇટ્રોજન ઘટકોના બંધક્રમાંકનો વધતો ક્રમ ક્યો છે ?View Solution
- 5$O_{2}^{2-}$ના તમામ બંધનીય આણ્વિય કક્ષકમાં ઇલેક્ટ્રોનની કુલ સંખ્યા $......$ છે.View Solution
- 6$SF_4 , CF_4$ અને $XeF_4$ પરમાણુ વિશે સાચુ વિધાન કયું છે?View Solution
- 7View Solutionઆપેલ ઘટકો વચ્ચેના પરમાણુ બળના આકર્ષણનો નીચેનામાંથી કયો ક્રમ ખોટો છે?
- 8View Solutionનીચે પૈકી કયું સહસંયોજક સંયોજન છે?
- 9જો ઈલેક્ટ્રોનિય ગોઠવણી $M = 2,\,\,8,\,\,3$ અને $A = 2,\,\,8,\,\,7,$ છે. તો નીચેના માંથી ક્યાં સંયોજન નું સૂત્ર બનશેView Solution
- 10View Solutionનીચેની પરમાણુની કઈ જોડીમાં સૌથી મજબૂત આંતરઆણ્વિય હાઇડ્રોજન બંધ હાજર છે ?
