નીચેનામાંથી કોણ મહત્તમ ઉત્ક્લનબિંદુ ધરાવે છે?
IIT 1986, Medium
b
(b)\( n-\) octane
(b)\( n-\) octane
* Boiling point depends on molecular mass. Greater the molecular mass higher will be the boiling point.
*Boiling point also depends on the structure. If two compounds have same molecular mass then straight chain or linear compound has higher boiling point.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેની પ્રક્રિયામાં $X$ શુ હશે ?View Solution
${C_2}{H_2}\xrightarrow[{HgS{O_4}/{H_2}S{O_4},60{\,^o}C}]{{{H_2}O}}X \rightleftharpoons C{H_3}CHO$
- 2View Solutionબેન્ઝિન કેવી રીતે પ્રાપ્ત થાય છે ?
- 3View Solutionપેટ્રોલિયમ મુખ્યત્વે શું ધરાવે છે ?
- 4$CH_3CH(Br)CH_2CH_3 + alc. KOH \to $ ?View Solution
- 5ઉપરોક્ત પ્રકિયા માં નિપજ $(B)$ શું છે ?View Solution
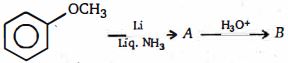
- 6View Solutionનીચેના પૈકી કોનો ઓક્ટેન આંક સૌથી વધુ છે ?
- 7$ n- $પ્રોપાઇલ બ્રોમાઇડની ઇથેનોલિક પોટેશિયમ હાઇડ્રોક્સાઇડ સાથે પ્રક્રિયા કરવા પર શું બનશે?View Solution
- 8View Solutionઆમાંના કયા પ્રતિ-માર્કોનિકોવના નિયમનું પાલન કરતા નથી
- 9સૂર્યપ્રકાશની હાજરીમાં $2-$મિથાઇલ બ્યુટેનની બ્રોમીન સાથેની પ્રક્રિયાથી મુખ્યત્વે ...... મળે છેView Solution
- 10$CH_3CH(Br)CH_2CH_3 + alc. KOH \to $ ?View Solution
