નીચેનામાંથી કયું સંકરણ બિન-રેખીય કક્ષકમાં પરિણમે છે
AIPMT 1991, Medium
a
Consider the geometry defined by the hybridization:-
Consider the geometry defined by the hybridization:-
$A)$ $sp$ - Linear
$B)$ $sp ^2$ - Trigonal planar
$C)$ $sp ^3$ - Tetrahedral
$D)$ $dsp$ - Square Planar.
Hence, $sp ^3$ hybridisation results in non-planar orbitals.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionસલ્ફયુરિક એસિડ અણુ ધરાવે છે .......
- 2$H_2O$ પાણી છે જ્યારે $H_2S$ ગેસ છે કારણ કેView Solution
- 3ક્યા આયન કે જેની (સ્પિન ફક્ત) ની ચુંબકીય ચાકમાત્રા $5.9\, BM$ હોય છે?View Solution
- 4View Solutionસ્ફટિકમાં ધનાયન અને ઋણાયન દ્વારા કયો બંધ રચાય છે?
- 5View Solutionરાસાયણિક બંધનને ધ્યાનમાં રાખીને નીચેના પરમાણુ માટે યોગ્ય વિકલ્પ પસંદ કરો
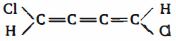
- 6ઇથીનના $\pi - $ બંધ માં નોડલ સમતલ ક્યાં ગોઠવાયેલ હશે?View Solution
- 7View Solutionસહસંયોજક સંયોજન માટે નીચેના માંથી ક્યુ વાકય સાચું છે
- 8વિધાન : સિગ્મા $(\sigma )$ પ્રબળ બંધ છે , જ્યારે પાઇ $(\pi )$ એ નિર્બળ બંધ છેView Solution
કારણ : અણુઓ $(\pi )$ બંધ વિશે મુક્તપણે ફરે છે. - 9નીચેનામાંથી કોણ $SiCl_4$ સાથે સમબંધારણીય નથી ?View Solution
- 10નીચે આપેલા પૈકી અનુચુંબકીય સ્પીસીઝોની સંખ્યા છે.View Solution
$B _{2}, Li _{2}, C _{2}, C _{2}^{-}, O _{2}^{2-}, O _{2}^{+}$ અને $He _{2}^{+}$
