The second ionization energy of sulphur is greater than that of chlorine because after removing of \(1^{st}\) electron it gets extra stability due to half-filled configuration.
But as after removal of the third electron, \(P\) gets extra stability due to full filled \(s\) orbitals so third ionization energy of phosphorus is lower than that of aluminium.
The first ionization energy of aluminium is approximately the same as that of gallium due to similar charge density.
The second ionization energy of boron is greater than that of carbon because in case of \(B\) and electron has to be removed from filled \(2 s\) orbitals.
Download our appand get started for free
Similar Questions
- 1અણુમાં ક્રમિક ઉર્જા સ્તર વચ્ચે ઉર્જા અંતર કેવી રીતે નીચીથી નીચી $n$ કિંમતોમાં બદલાય છે?View Solution
- 2$Mg$ કરતા $Al$ ની પ્રથમ આયનીકરણ એન્થાપી ઓછી છે, કારણ કે .......................View Solution
- 3View Solutionનીચેના પૈકી ક્યુ વિધાન સાચું છે ?
- 4$Ar, K^+$ અને $Ca^{2+}$ ઘટકો સમાન સંખ્યાના ઇલેક્ટ્રોન ધરાવે છે. તો તેઓની ત્રિજ્યા ક્યા ક્રમમાં વધશે ?View Solution
- 5સોડિયમ સામાન્ય રીતે $ + 2$ ની ઑક્સિડેશન સ્થિતિ બતાવતું નથી કારણકેView Solution
- 6View Solutionઆયનીકરણ શક્તિના નીચેના ક્રમમાંથી કયો ક્રમ સાચો છે?
- 7તત્વ $Z=114$ હાલમાં જ શોધાયુ છે. તેનો સમાવેશ નીચેના પૈકી ક્યા family $/$ સમૂહમાં થશે અને તેની ઇલેક્ટ્રોન રચના શુ થશે ?View Solution
- 8View Solutionનીચેનામાંથી ક્યુ દર્શાવેલ ગુણધર્મોનો સાચો ક્રમ દર્શાવતુ નથી ?
- 9નીચેના તત્વોને ધ્યાનમાં લો. સમૂહ. તો $A'$, $B'$, $C'$ અને $D'$ માટે યોગ્ય વિધાન શોધો ?View Solution
$A.$ પરમાણુ ત્રિજ્યાનો ક્રમ: $\mathrm{B}^{\prime}<\mathrm{A}^{\prime}<\mathrm{D}^{\prime}<\mathrm{C}^{\prime}$
$B.$ ધાત્વીય લક્ષગનો ક્રમ: $\mathrm{B}^{\prime}<\mathrm{A}^{\prime}<\mathrm{D}^{\prime}<\mathrm{C}^{\prime}$
$C.$ તત્વનાકદનો ક્રમ: $\mathrm{D}^{\prime}<\mathrm{C}^{\prime}<\mathrm{B}^{\prime}<\mathrm{A}^{\prime}$
$D.$ આયોનીક ત્રિજ્યાનો ક્રમ: $\mathrm{B}^{\prime+}<\mathrm{A}^{\prime}+<\mathrm{D}^{\prime}+<\mathrm{C}^{\prime}+$
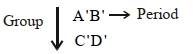
- 10View Solutionસૌથી વધુ આયનીકરણ એન્થાલ્પી ધરાવતુ ઇલેક્ટ્રોનીય બંધારણ જણાવો.
