Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેના સમૂહોમાં....... છુટા પડતા સમૂહની ક્ષમતાનો ક્રમ કયો છે ?View Solution
$I$ $ -OAc$
$II$ $-OMe$
$III$ $-OSO_2Me$
$IV$ $OSO_2CF_3$
- 2View Solutionનીચે આપેલ જુથ માંથી મેટા નિર્દેશક ક્રિયાશીલ સમૂહોનું જૂથ શોધો.
- 3View Solutionનીચેના સંયોજનોમાં ઇલેક્ટ્રોન અનુરાગી વિસ્થાપન પ્રક્રિયાઓમાં પ્રતિક્રિયાશીલતાનો યોગ્ય ક્રમ હશે
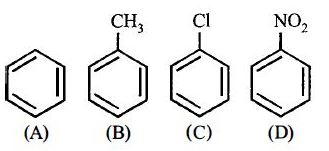
- 4View Solutionનીચેના પૈકી ક્યુ સંયોજન મહતમ દ્વિધુવ ચાકમાત્રા દર્શાવશે ?
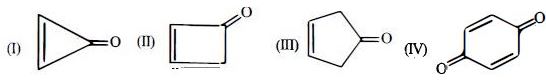
- 5$O{H^ - },\,\,NH_2^ - \,,HC\,\, \equiv \,\,{C^ - }\,,\,\,C{H_3}\, - \,\,CH_2^ - $ બેઈઝની પ્રબળતાનો ઘટતો ક્રમ કયો છે ?View Solution
- 6${(C{H_3})_3}\overline C \,,\,\,\overline C \,C{l_3}\,,\,\,{(C{H_3})_2}\,\overline C H\,,\,\,{C_6}{H_5}\,\overline C {H_2}$ આ કાર્બેનાયન ને તેમની ઘટતી સ્થિરતા ના ક્રમમાં ગોઠવવો.View Solution
- 7આપેલા સંયોજનો $CH \equiv CH,\,C{H_3} - C \equiv CH,\,$ અને $C{H_2} = C{H_2}$ માટે તેમના એસિડ શક્તિનો સાચો ક્રમ નીચેનામાથી કયો થશે?View Solution
- 8નીચેનામાંથી કયો સેન્ડ મેયર પ્રકિયા માં મધ્યવર્તી શું હશે ?View Solution
$(i)$ ${C_6}{H_5}{N^ + } \equiv NC{l^ - }$ $(ii)$ ${C_6}{H_5}{N^ + } \equiv N$
$(iii)$ ${{\overset{\centerdot }{\mathop{C}}\,}_{6}}{{H}_{5}}$ $(iv)$ $C_6H_5Cl$ - 9$\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CHO}$ ના નીચે આપેલા સસ્પંદન બંધારણો નો સાચો સ્થિરતા ક્રમ શાધોView Solution
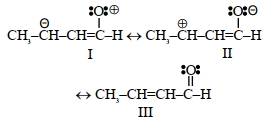
- 10View Solutionનીચેના મુક્તમૂલકોની સ્થાયીતાનો સાચો વધતો ક્રમ .....
