નીચેનામાંથી મુકતમુલક નો સ્થાયિતાનો વધતો ક્રમ કયો છે?
AIIMS 2017, Diffcult
b
The order of stability of free radicals
The order of stability of free radicals
\({({C_6}{H_5})_3}\dot C\, > \,{({C_6}{H_5})_2}\dot CH\, > \,{(C{H_3})_3}\dot C\, > \,{(C{H_3})_2}\dot CH\)
The stabilisation of first two is due to resonance and last two is due to inductive effect.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી કયો પ્રબળ બેઈઝ છે ?
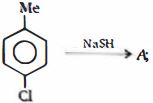
- 2પ્રકિયા ની નીપજ $(A)$ શું હશેView Solution
- 3View Solutionપ્રેરક અસર અંગે નીચેનામાંથી કયો ક્રમ સાચો છે?
- 4View Solutionઉપરોક્ત સંયોજનમાં હાજર મોટાભાગના એસિડિક હાઇડ્રોજનને ઓળખો
- 5$(I)$ $CH_3CH_2COOH $View Solution
$(II)$ $CH_2 = CH - COOH $ અને
$(III)$ $ HC \equiv C - COOH$
એસિડોની ગોઠવણી ઘટતી એસિડીકતા ક્રમમાં ગોઠવો.
- 6બેઇઝ પ્રબળતાના ઘટતા ક્રમમાં નીચેના ક્રમ આપોView Solution
$(A)\, CH_3 - CH_2 - C \equiv C^-$ $(B) \,CH_3 -CH_2 - S^-$
$(C) \,CH_3 - CH_2 - CO^-_2$ $(D)\, CH_3 -CH_2 - O^-$ - 7View Solutionકયો ખોટો સ્થિરતા ક્રમ છે ?
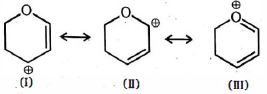
- 8View Solutionઆપેલ બંધારણ વચ્ચે સૌથી સ્થાયી પ્રમાણભૂત બંધારણ કયું છે
- 9View Solutionકોના દ્વારા ડાયપોલ મોમેન્ટ જોવા મળે છે ?
- 10પ્રક્રિયા $CH_3Br+Nu^- \rightarrow CH_3 -Nu + Br^-$ માટે કેન્દ્રઅનુરાગી $(Nu^-)$ $A$ થી $D$ ના પ્રક્રિયા દરનો ધટતો ક્રમ જણાવોView Solution
$[Nu^-=(A) \,PhO^-, (B)\,AcO^-, (C)\, HO^-, (D)\, CH_3O^-]$
