નીચેનામાંથી $SO_3$ માટે કયું બંધારણ સૌથી વધુ પસંદ થયેલ છે અને તેથી સૌથી ઓછી ઊર્જા છે?
AIPMT 2011, Advanced
d
Formal charges help in selection of the lowest energy structure from a number of possible Lewis
Formal charges help in selection of the lowest energy structure from a number of possible Lewis
structures for a given species. Generally the lowest energy structure is the one with the smallest
formal charges on the atoms.
Formal charge on an atom $=$ total no. of valence electrons - non-bonding electrons $-\frac{1}{2} \times$ bonding
electrons.
For Lewis structure of $\mathrm{SO}_{3}$
Formal charge on S atom $=6-0-\frac{1}{2} \times 12=0$
Formal charge on three O atoms
$=6-4-\frac{1}{2} \times 4=0$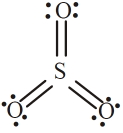
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચેનામાંથી કઈ ઘટકએ કેન્દ્રિય અણુના સંકરણમાં $d-$ભ્રમણકક્ષાના બંને અક્ષીય સેટનો ઉપયોગ કર્યો છે?View Solution
- 2View Solutionઈથેનોલ અને ડાયમિથાઈલ ઈથર ફંકશનલ આઈસોમર ની જોડી બનાવે છે. ઈથેનોલ નું ગલન બિંદુ ડાયમીથાઈલ ઈથર ની તુલના માં ઊંચું છે કારણ કે
- 3સમાન અણુના સંકર કક્ષકોના આપેલા ક્રમ માટે લાક્ષણિકતાઓનો યોગ્ય કોડ પસંદ કરો,View Solution
$sp < sp^2 < sp^3$
$(i)$ વિદ્યુતઋણતા $(ii)$ સમાન સંકર કક્ષકો વચ્ચેનો બંધકોણ$(iii)$ કદ $(iv)$ ઉર્જા સ્તર
- 4$N_2$ ના દરેક બાહ્ય કૌસ દ્વારા વહેચાયેલ ઈલેક્ટ્રોનની સંખ્યા શું હશેView Solution
- 5વિધાન : સિગ્મા $(\sigma )$ પ્રબળ બંધ છે , જ્યારે પાઇ $(\pi )$ એ નિર્બળ બંધ છેView Solution
કારણ : અણુઓ $(\pi )$ બંધ વિશે મુક્તપણે ફરે છે. - 6View Solutionનીચેનામાંથી ક્યો ધટક પિરામિડ આકાર ધરાવે છે?
- 7${H_2}O,N{H_3},C{H_4},C{O_2}$ માં બંધ ખૂણાનો સાચો ક્રમ નીચેનામાંથી ક્યો હશે?View Solution
- 8View Solutionશૂન્ય દ્વિધ્રુવીય ચાકમાત્રા સાથે ઝેનોન ધરાવતું સંયોજન કયું છે?
- 9View Solutionઓક્સિજનની ઘટકોની જોડી અને તેના ચુંબકીય વર્તન નીચે નોંધવામાં આવે છે. નીચેનામાંથી કયો વિકલ્પ સાચા વર્ણન રજૂ કરે છે?
- 10જ્યારે કાર્બન અણુની સંકરણ સ્થિતિ $s{p^3}$ થી $s{p^2}$ થી $sp,$ માં બદલાય છે, સંકર કક્ષક વચ્ચેનો કોણ શું હશે ?View Solution
