\(A.\) the polarity increasing in the order is \(HI\, <\, HBr\, < \,HCl\, <\, HF\)
, as suggested by the electronegativity differences. \(H - F\) is more polar than \(HBr\), therefore option \(A\) is wrong as it say \(HF\) is less polar than \(HBr\).
\(B.\) Absolutely pure water does not contain any ions is false because pure water, has an amphiprotic nature. This means that a small amount of ions will form in pure water.
\(C.\) Chemical bond formation take place when force of attraction overcome the forces of repulsion is true because the bond is result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds.
\(D.\) In co-valency transference of electron takes place is wrong because he formation of an Ionic bond is the result of the transfer of one or more electrons from a metal onto a non-metal.
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાંથી કઇ જોડ સમબંધારણીય છે ?
- 2$sp$ સંકરણ ના પરિણામે આપણે મેળવીએ છીએ કેView Solution
- 3નીચેના માંથી શેમાં $p\pi - d\pi $ બંધ હોય છેView Solution
- 4$CS_2$ માટે સૌથી વધારે સ્થાયી લુઇસ બંધારણ વિશે કયુ વિધાન સાચું છે?View Solution
- 5View Solutionઆયનિક સંયોજનની લેટાઇસ શક્તિનો આધાર નીચેનામાંથી કોની ઉપર હોય?
- 6નીચેનામાંથી ક્યુ સંયોજન ધુવીય છે અને તેમાં મધ્યસ્થ પરમાણુ $sp^2$ સંકરણ ધરાવે છે ?View Solution
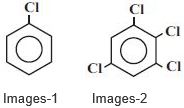
- 7$Images-1$ ની દ્વિધુવ ચાકમાત્રા $1.5 \,D$ હોય, તો $Images-2$ ની દ્વિધુવ ચાકમાત્રા .................. $\mathrm{D}$ થશે.View Solution
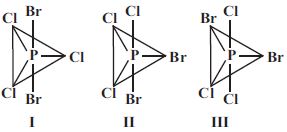
- 8$PCl_3Br_2$ ભૌમિતિક સમઘટકતા દર્શાવે છે, જેના ભૌમિતિક સમઘટકો નીચે મુજબ છે. તે પૈકી કોણ દ્વિધ્રુવ ચાકમાત્રા ધરાવતો(તા) નથી ?View Solution
- 9$N_2, O_2, O_2^-$ પૈકી બંધઉર્જાનો સાચો ક્રમ નીચેના દર્શાવેલી કઈ ગોઠવણીમાં છે ?View Solution
- 10View Solutionનીચેનામાંથી ક્યા સંયોજનમાં બે સહસંયોજક બંધો વચ્ચે સૌથી મોટો ખૂણો હશે?
