નીપજ $(A)$ શું હશે ?
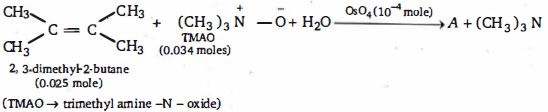
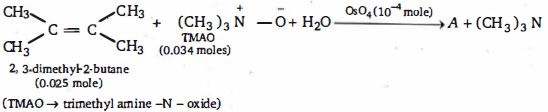
Medium
b
$(b)$ Once a small amount of $OsO_4$ is used up the $Os \,(VI)$ by product is oxidized with is the reaction mixture by the amine oxide to re-form $OsO_4$, thus, a catalytic amount of $OsO_4$ can be used and the amine oxide acts as the ultimate oxid
$(b)$ Once a small amount of $OsO_4$ is used up the $Os \,(VI)$ by product is oxidized with is the reaction mixture by the amine oxide to re-form $OsO_4$, thus, a catalytic amount of $OsO_4$ can be used and the amine oxide acts as the ultimate oxid
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચે આપેલા સંયોજન $(3)$ ને બાકીના સંયોજનોથી અલગ પાડવા માટે સૌથી યોગ્ય પ્રક્રીયક ક્યો છે ?View Solution
$(1)$ $CH_3 - C \equiv C - CH_3$ $(2)$ $CH_3CH_2CH_2CH_3$
$(3)$ $CH_3CH_2C \equiv CH$ $(4)$ $CH_3CH = CH_2$
- 2$n-$પ્રોપાઇલ બ્રોમાઇડની ઇથેનોલીક પોટેશિયમ હાઇડ્રોકસાઇડ સાથેની પ્રક્રિયાથી કઇ નીપજ મળે છે ?View Solution
- 3નીચેની પ્રક્રિયામાં મુખ્ય નીપજ ($Y$) શું હશે ?View Solution
$\begin{array}{*{20}{c}}
{C{H_3}\,\,\,\,\,\,\,\,\,} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_3} - CH - C = CH}
\end{array}\xrightarrow[{{H_2}O}]{{HgS{O_4},{H_2}S{O_4}}}X$ $\xrightarrow[{(ii)\,conc.{H_2}S{O_4}/\Delta }]{{(i)\,{C_2}{H_5}MgBr,{H_2}O}}Y$ - 4$HBr$ તરફ ની પ્રતિક્રિયાશીલતાના ઘટતા ક્રમમાં ઉપરની ગોઠવણી કરોView Solution
- 5$FeCl_3$ ની હાજરીમાં બેન્ઝિનના ક્લોરિનેશન માટે જરૂરી ઇલેક્ટ્રોન અનુરાગી આયન..... છે.View Solution
- 6નીચેનામાંથી કઈ પ્રક્રિયા ફ્રીડલ-ક્રાફ્ટ્ આલ્કાઈલેશન પ્રક્રિયામાં મુખ્ય નીપજ તરીકે $2-$ફિનાઇલબ્યુટેન આપશે નહીં ?View Solution
- 7View Solutionએસિટિલિન .... આપે છે.
- 8$C_6H_{10}$ અણુસૂત્ર ધરાવતો હાઇડ્રોકાર્બન ફક્ત એક જ $H_2$ અણુનુ શોષણ કરે છે. હાઇડ્રોકાર્બનના ઓઝોનોલિસિસથી $OHC-CH_2CH_2CH_2CH_2CHO$ મળે છે. તો તે હાઇડ્રોકાર્બન ........ હશે.View Solution
- 9View Solutionનીચેના પૈકી ક્યુ સંયોજન સરળતાથી ફ્રિડલ-ક્રાફ્ટ પ્રક્યિા આપશે નહિ ?
- 10હેલોહાઈડ્રીન રચવા માટે સાયક્લોહેક્સિનમાં પાણીમાં $Cl_2$ ના ઇલેક્ટ્રોઅનુરાગી ઉમેરામાં મધ્યવર્તીનું સૌથી સ્થિર સ્વરૂપ કયું છે ?View Solution
