(b) \(\begin{array}{*{20}{c}}
{\,\,\,\,\,\,C{H_3}} \\
| \\
{C{H_3} - C - C{H_2}} \\
{|\,} \\
{\,\,\,\,\,\,C{H_3}}
\end{array} + \mathop {KOH}\limits_{(alc)} \to \) \(\begin{array}{*{20}{c}}
{\,\,C{H_3}\,\,\,\,\,\,\,\,\,\,\,\,} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_3} - C = CH - C{H_3}}
\end{array} + KBr + {H_2}O\)
In this reaction \({1^o}\) carbonium ion is formed which rearranges to form \({3^o}\) carbonium ion from which base obstruct proton. Hence \(2-\) methyl-\(2\) -butene is formed as a main product.
\(\mathop {\begin{array}{*{20}{c}}
{\,\,\,\,\,\,C{H_3}} \\
| \\
{C{H_3} - C - \mathop C\limits^ + {H_2}} \\
{|\,} \\
{\,\,\,\,\,\,C{H_{3\,\,}}}
\end{array}}\limits_{{1^o}\,carbonium\,less\,stable} \) \(\xrightarrow{{Methyl\,shift}}\begin{array}{*{20}{c}}
{C{H_{3\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{\mathop {C{H_3} - C - C{H_2} - C{H_3}}\limits_ + }
\end{array}\)
\(\xrightarrow{{Elimination{\kern 1pt} of{\kern 1pt} proton{\kern 1pt} from{\kern 1pt} \beta {\kern 1pt} carbon{\kern 1pt} which{\kern 1pt} is{\kern 1pt} less{\kern 1pt} hydrogenated}}\mathop {\begin{array}{*{20}{c}}
{\,C{H_{3\,\,\,\,\,\,\,\,\,\,\,\,\,\,\;}}} \\
{\,\,\,\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{C{H_3} - C = CH - C{H_3}}
\end{array}}\limits_{2 - Methyl - 2 - Butene} \)
Download our appand get started for free
Similar Questions
- 1View Solutionનીચેનામાંથી કોણ વુર્ટઝ-ફીટિગ પ્રક્રિયા રજૂ કરે છે?
- 2View Solutionકેલ્શિયમ કાર્બાઇડની ભારે પાણી સાથેની પ્રક્રિયાથી શું મળે છે ?
- 3View Solutionટોલ્યુઈનનું.......સાથેના ઓક્સિડેશન કરવાથી બેન્ઝાલ્ડિહાઇડમાં રૂપાંતર થાય છે.
- 4નીચે આપેલા સંયોજન $(3)$ ને બાકીના સંયોજનોથી અલગ પાડવા માટે સૌથી યોગ્ય પ્રક્રીયક ક્યો છે ?View Solution
$(1)$ $CH_3 - C \equiv C - CH_3$ $(2)$ $CH_3CH_2CH_2CH_3$
$(3)$ $CH_3CH_2C \equiv CH$ $(4)$ $CH_3CH = CH_2$
- 5$KOH$ નું આલ્કોહોલીક દ્રાવણ એ કોનો વિશિષ્ટ પ્રક્રિયક છે ?View Solution
- 6View Solutionઓલેફિન બંધમાં મિનરલ એસિડનો સમાવેશ મુખ્ય નીપજ તરફ લઈ જાય છે, તેને ઓળખો
- 7View Solutionજ્યારે મિથેન અને ઓક્સિજનના મિશ્રણને ગરમ કરેલ મોલિબ્ડેનમ ઓક્સાઇડ પરથી પસાર કરવામાં આવે ત્યારે મળતી નીપજ ........ છે.
- 8આલ્કાઇન $KMnO_4$ દ્રાવણનો રંગ દૂર કરતું નથી તથા ઍમોનિયમ સિલ્વર નાઇટ્રેટ સાથે અવક્ષેપ આપતું નથી આ હાઇડ્રોકાર્બન ....... છે.View Solution
- 9પ્રક્રિયા સાથે ઉદીપક જોડો,View Solution
ઉદીપક પ્રક્રિયા $(i) \;\mathrm{Na}_{2} \mathrm{O}$ $(a)$ ઇથાઈન માથી ઇથેનાલમાં ઓક્સિડેશન $(ii) \;\mathrm{TiCl}_{4}+ \mathrm{Al(CH_3)}_{3}$ $(b)$ આલ્કાઇન્સનું બહુલીકરણ $(iii)\;\mathrm{PdCl_2} $
$(c)$ $H_2SO_4$ની બનાવટમાં $SO_2$ના ઓક્સિડેશનમાં $(iv)\;$ નિકલ સંકીર્ણો $(d)$ ઇથિલીનનું બહુલીકરણ નીચેના પૈકી કયો વિકલ્પ સાચો છે?
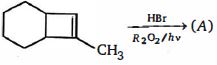
- 10નીપજ $(A)$ શું હશે ?View Solution