ઓર્થો-નાઇટ્રોફિનોલ એ $p-$ અને $m-$ નાઇટ્રોફિનોલ્સ કરતાં પાણીમાં ઓછું દ્રાવ્ય છે કારણ કે:
AIEEE 2012, Medium
b
Due to intramolecular H-bonding, - OH group is not available to form a hydrogen bond with water. Hence o-nitrophenol is sparingly soluble in water while $\mathrm{m}$ - and p-nitrophenol are soluble due to intermolecular H-bonding with water.
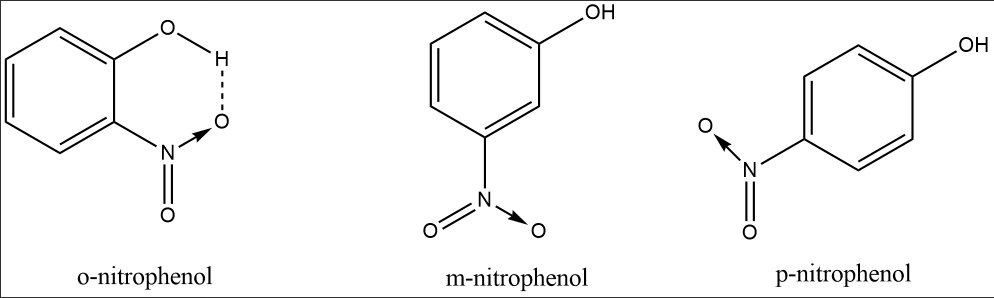
Due to intramolecular H-bonding, - OH group is not available to form a hydrogen bond with water. Hence o-nitrophenol is sparingly soluble in water while $\mathrm{m}$ - and p-nitrophenol are soluble due to intermolecular H-bonding with water.

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચે આપેલામાંથી અણુઓ અથવા આયનોની સંખ્યા કે જે ઈલેકટ્રોનોની એકી સંખ્યા ધરાવતા નથી તે $.........$View Solution
$(A)$ $NO _2$ $(B)$ $ICl _4^{-}$ $(C)$ $BrF _3$ $(D)$ $ClO _2$ $(E)$ $NO _2^{+}$ $(F)$ $NO$
- 2$AsF_3Cl_2$માં $\angle \,FAsF$ બંધકોણ ......View Solution
- 3$NO_3^-,NO_2^+$ અને $NH_4^+$ માં $N$ પમાણુની કક્ષકોનુ સંકરણ અનુક્રમે ... છે.View Solution
- 4View Solutionનીચે આપેલ જોડીમાંથી કયા બે ઘટકો સમબંધારણીય નથી?
- 5View Solutionકેમા મહત્તમ બંધ ખૂણો હાજર છે
- 6View Solutionહાઇડ્રોજન પરમાણુ એકબીજા સાથે જોડાઈને હાઇડ્રોજન બંધ બનાવે છે તો તે નીચેના માંથી ક્યાં બંધ દ્વારા બનાવે છે
- 7View Solutionનીચેનામાંથી કયા સંયોજન માં આયનીય તેમજ સહસંયોજક બંધનો સમાવેશ થાય છે ?
- 8View Solutionનીચે આપેલા તત્વ માંથી ક્યુ તત્વ આઠ પરમાણુનું અણુ બનાવે છે
- 9પાણીના અણુમાં બે બંધ વચ્ચેનો ખૂણો ............... $^o$ હોય છે?View Solution
- 10$KO_2, AlO^-_2, BaO_2$ અને $NO^+_2$ પૈકી કોણ અયુગ્મિત ઇલેક્ટ્રોન ધરાવે છે?View Solution
