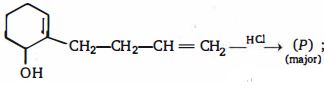Download our appand get started for free
Similar Questions
- 1View Solutionનીચેની પ્રકીયામાંથી સાચી નીપજ કઈ છે ?
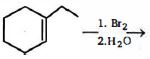
- 2View Solutionનીચેની પ્રકિયામાંથી સાચી નીપજ કઈ છે ?
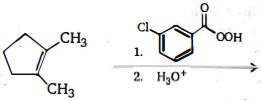
- 3નીચેનામાંથી એવી પ્રકિયા પસંદ કરો કે જે $2$ -બ્રોમોપ્રોપેન ના ઉત્પાદન માં વપરાય છેView Solution
$(I) \,C{H_3}CH = C{H_2} + HBr\xrightarrow{{peroxide}}$
$(II)\, C{H_3}CH = C{H_2} + HBr\xrightarrow{{CC{l_4}}}$
$(III)\, C{H_3}C{H_2}C{H_3} + B{r_2}\xrightarrow{{hv}}$
$(IV)\,C{H_3}CH = C{H_2} + B{r_2}\xrightarrow{{CC{l_4}}}$
- 4ઇથાઇનને $HgSO_4$ અને મંદ $H_2SO_4$ ની હાજરીમાં $333\,K$ તાપમાને ગરમ કરતાં મળતી અંતિમ નીપજ......... છે.View Solution
- 5View Solutionજ્યારે ટોલ્યુઈનને પ્રકાશની હાજરીમાં અને હેલોજન કેરીયરની ગેરહાજરીમાં ક્લોરીન સાથે ગરમ કરવામાં આવે તો શું ઉત્પન્ન થાય ?
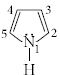
- 6View Solutionપાયરોલમાં ક્યાં ઇલેક્ટ્રોનની ઘનતા મહત્તમ છે
- 7નીચે આપેલી પ્રકિયા માં નીપજ શું હશે ?View Solution
$\begin{array}{*{20}{c}}
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,OH} \\
{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|} \\
{C{H_3}{\text{ }} - {\text{ }}CH{\text{ }} = {\text{ }}CH{\text{ }} - {\text{ }}C{H_3}\xrightarrow{{{H_3}{O^ + }}}\,C{H_3} - C{H_2} - CH - C{H_3}}
\end{array}$ - 8નીચે બતાવેલ $3,3$ - ડાયમિથાઇલ - $1$ -બ્યુટીન ના વધારાને ધ્યાનમાં લો.નિરીક્ષણ કરેલ નીપજ ની રચના માટે શ્રેષ્ઠ પદ્ધતિઓની સમજૂતી શું છે?View Solution
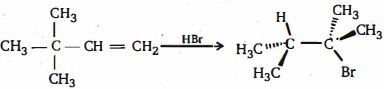
- 9View Solutionઈથાઈલ એસિટેટનું પાયરોલીસીસ શું આપે છે ?
- 10નીપજ $(P)$ શું હશે ?View Solution