પેરોક્સાઇડની હાજરીમાં, $HCl$ અને $HI$ એ આલ્કીન સાથે પ્રતિમાર્કોનિકોવ નિયમ મુજબ યોગશીલ પ્રક્રિયા આપતા તથી, કારણ કે .........
JEE MAIN 2014, Diffcult
a
Anti-Markownikoff addition is possible only in case of \(HBr\) and not in \(HCl\) and \(HI\) . In \(HBr\) both the chain initiation and propagation steps are exothermic, while in \(HCl,\) first step is exothermic, and second step is endothermic and in \(HI,\) no step is exothermic. Hence \(HCl\) and \(HI\) do not undergo anti-Markownikoff's addition.
Anti-Markownikoff addition is possible only in case of \(HBr\) and not in \(HCl\) and \(HI\) . In \(HBr\) both the chain initiation and propagation steps are exothermic, while in \(HCl,\) first step is exothermic, and second step is endothermic and in \(HI,\) no step is exothermic. Hence \(HCl\) and \(HI\) do not undergo anti-Markownikoff's addition.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionઆલ્કેેન માટે કયું વિધાન ખોટું છે.
- 2View Solutionસાંદ્ર નાઇટ્રીક એસિડ અને સાંદ્ર સલ્ફયુરિક એસિડ સાથે બેન્ઝિનના નાઇટ્રેશનમાં કયા સક્રિય ધટક સંકળાયેલ છે
- 3$2$ -હેપ્ટેનોન તૈયાર કરવા માટે કઈ પ્રક્રિયાઓનો ક્રમ શ્રેષ્ઠ છે.View Solution
- 4બેન્ઝિનની $CH_3COCl$ સાથે $AlCl_3$ ની પ્રક્રિયાથી મળતિ નીપજ ..... છે.View Solution
- 5નીચેના પૈકી ક્યો $o-/ - p -$ નિર્દેશક સમૂહ છે ?View Solution
- 6View Solutionપ્રક્રિયાઓની કઈ શ્રેણી નીચેના પરિવર્તનને પ્રાપ્ત કરશે?
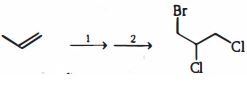
- 7View Solutionએરોમેટિક કેન્દ્રના ક્લોરિનેશનમાં ક્યો ઘટક ઉત્પન્ન થાય છે ?
- 8View Solutionપ્રોપાઈન અને પ્રોપીન ને કઈ રીતે અલગ કરી શકાય છે ?
- 9$H_2\, (Pd/C)$ સાથે આપેલ પ્રકીયકો ના ઉત્પ્રેરક હાઇડ્રોજનરેશન પર ઉષ્ણતામાનનો ઘટાડો થયો એનો ક્રમ ક્યો છે ?View Solution
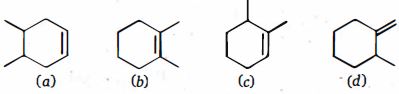
- 10View Solutionઆપેલ આલ્કિનના હાઇડ્રોબોરેશન -ઑક્સિડેશન પર પ્રાપ્ત નિપજો શું છે?