(b) The formation of $n-$propyl bromide in presence of peroxide can be explained as follows.
Step$-1:$ Peroxide undergo fission to give free radicals $R - O - O - R \to 2 - R - \dot O$
Step$-2 :$ $HBr$ combines with free radical to form bromine free radical $R - \dot O + HBr \to R - OH + B\dot r$
Step$-3 :$ $B\dot r$ attacks the double bond of the alkene to form a more stable free radical
Step$-4 :$ More stable free radical attacks the $HBr$
$C{H_3} - \dot CH - C{H_2} - Br + HBr \to \mathop {C{H_3}C{H_2}C{H_2}Br}\limits_{{\rm{n - propyl bromide}}} + B\dot r$
Step$-5 :$ $B\dot r + B\dot r \to B{r_2}$
$C{H_3}CH = C{H_2} + Br \to \mathop {C{H_3} - CH - C{H_2}Br}\limits_{(more\,stable)} $
$C{H_3}CH = C{H_2} + Br \to \mathop {\begin{array}{*{20}{c}}
{Br} \\
| \\
{C{H_3} - CH - C{H_2}}
\end{array}}\limits_{(less\,stable)} $
Download our appand get started for free
Similar Questions
- 1આલ્કેન સંયોજનોમાં $C - H$ બંધલંબાઇ, $H - C - H$ બંધકોણ અનુક્રમે...... હોય છે.View Solution
- 2નીચેનામાંથી ઓઝોનોલિસીસના ઉત્પાદન તરીકે કયા સંયોજનો એસીટોન $(CH_3)_2 C = O$આપે છે ?View Solution
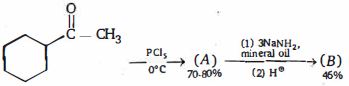
- 3નીપજ $(B)$ શું છે ?View Solution
- 4$3$-ફિનાઈલ પ્રોપીન પર $HBr$ સાથે પ્રક્રિયા કરતા (મુખ્ય નીપજ) શું મળે છે ?View Solution
- 5આપેલી પ્રકિયા ની મુખ્ય નીપજ કઈ હશે ?View Solution
$H_2C = CH - CH_2 - I \xrightarrow[CC{{l}_{4}}]{HI(excess)}$
- 6View Solutionનીચેનામાંથી એરોમેટિક કોણ નથી ?
- 7View Solutionનીચેના પૈકી કોની હેલોજન એસિડ સાથેની પ્રક્રિયા સૌથી ધીમા દરથી થશે ?
- 8નીચેનામાંથી ક્યાં સંયોજનને પ્રબળ એસિડ સાથે ગરમ કરતાં $2,{3-}$ડાયમિથાઇલ$-{2-}$બ્યુટીન બનાવી શકાય છે?View Solution
- 9View Solutionબેયરનો પ્રક્રિયક ....
- 10View Solutionનીચે આપેલા પરમાણુઓના હાયડ્રોજીનેશનની ઉષ્માનો યોગ્ય ક્રમ કયો છે ?