Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$1$-બ્યુટાઈનનું ઓક્સિમરક્યુરેશન $(HgSO_4 + H_2SO_4) $ દ્વારા કઈ નીપજ મળશે ?View Solution
- 2View Solutionનીચે આપેલા સંયોજનોમાંથી કયો એક અચક્રીય છે?
- 3નીપજ $(P)$ શું હશે ?View Solution
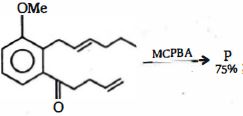
- 4નીચેના પૈકી કયા સંયોજનમાં રહેલ દરેક $C - C$ બંધલંબાઇ સમાન છે. ?View Solution
- 5નીચે આપેલા પરિવર્તન માટે પ્રક્રિયકનો સાચો સેટ (જોડી) પસંદ કરો.View Solution
ટ્રાન્સ $\left( Ph - CH = CH - CH _3\right) \rightarrow$ સીસ $\left( Ph - CH = CH - CH _3\right)$
- 6View Solutionઆલ્કેનનુ પ્રકાશરાસાયણિક ક્લોરિનેશન ..... દ્વારા શરૂ થાય છે.
- 7View Solutionએરોમેટિક સંયોજનોના નાઈટ્રેશન પર નીચેના વિધાનોમાં કયુ ખોટું છે?
- 8View Solutionક્યા પ્રક્રિયકોની શ્રેણી દ્વારા એસિટિલિનનું ઉચ્ચ આલ્કાઇનમાં રૂપાંતર કરી શકાય છે ?
- 9પોલિનેન્સમાં જેમાં અલગથી અવેજી$(C=C)$ દ્વિ-બંધ છે રસાયણિક રીતે એક$(C=C)$ દ્વિ-બંધ હાઈડ્રોજન બનાવવાનું શક્ય છે તો નીપજ કઈ હશે ?View Solution
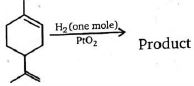
- 10View Solutionજંતુનાશક ગૈમેક્સેનનું રાસાયણિક નામ શું છે?
