સૌથી મોટી $C-H$ બંધ-લંબાઈ ક્યા સંયોજનમાં છે?
IIT 1989, Medium
c
Bond length is defined as the distance between two atoms that are participating in a bond.
Bond length is defined as the distance between two atoms that are participating in a bond.
We can also understand the bond length with the help of the \(s\) character.
Higher the \(s\)-character lesser is the bond length.
This is because higher \(s\) character implies that the electrons that are bonded pairs are closely held together, thus making the bond length small as well as strong.
Hybridisation of \(C _2 H _6\) is \(sp ^3\)
The \(s\) character in \(C _2 H _6\) (Ethane) is: \(\frac{1}{4} \times 100=25 \,\%\) which is the least amongst the other given compounds.
Therefore the \(C - H\) bond length of \(C _2 H _6\) is the longest.
Hence it is the correct option.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચે પૈકી કયો બંધ ધરાવતો હાઇડ્રોકાર્બન સૌથી વધુ ક્રિયાશીલ છે?
- 2View Solutionનીચે આપેલ પરિવર્તન માટે સાચું વિધાન ઓળખો.
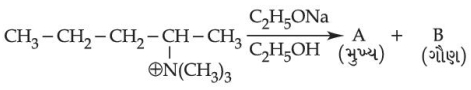
- 3ઉપરોક્ત રૂપાંતર ને બનાવવા માટે $(A)$ અને $(B)$ શું હશે ?View Solution
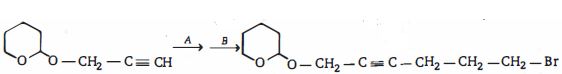
- 4View Solutionએસિટીલીન એ હાઈપોક્લોરસ એસિડ સાથે પ્રક્રિયા કરે તો નીપજ શું હશે ?
- 5$CH _3\left( CH _2\right)_4 CH _3 \xrightarrow[HCl,\Delta]{Anhy.AlCl_3} X$ આપેલ પ્રક્રિયામાં મુખ્ય નીપજ $X$ શોધો.View Solution
- 6View Solutionઘન મિથેન એ .....
- 7જ્યારે પ્રોપાઇનની $H_gSO {_4}$ની હાજરીમાં જલીય ${H_2} S {O_4}$ સાથે પ્રક્રિયા થાય છે, ત્યારે મુખ્ય નીપજ કઈ બને છે?View Solution
- 8View Solutionસાદામાં સાદો આલ્કાઇન..... દ્વારા દર્શાવાય છે ?
- 9$1,2$-ડાયબ્રોમો ઇથેનની આલ્કોહોલિક પોટાશ સાથેની પ્રક્રિયાથી....... મળે છે.View Solution
- 10View Solutionપ્રોપીનમાંથી પ્રોપેન કઈ પદ્ધતિ દ્વારા મેળવવામાં આવે છે?
