સૂર્યપ્રકાશમાં $C{H_4}$ની $C{l_2}$ની પ્રક્રિયા દ્વારા નીચે પૈકી કયુ બનતું નથી?
AIIMS 1987, Medium
d
(d) \(C{H_4}\xrightarrow{{C{l_2}}}C{H_3}Cl\xrightarrow{{C{l_2}}}C{H_2}C{l_2}\xrightarrow{{C{l_2}}} \,\,CHC{l_3}\xrightarrow{{C{l_2}}}CC{l_4}\).
(d) \(C{H_4}\xrightarrow{{C{l_2}}}C{H_3}Cl\xrightarrow{{C{l_2}}}C{H_2}C{l_2}\xrightarrow{{C{l_2}}} \,\,CHC{l_3}\xrightarrow{{C{l_2}}}CC{l_4}\).
Since this reaction takes place by free radical mechanism. Hence, there is a possibility of formation of ethane.
\(\mathop {\mathop C\limits^. {H_3} + \mathop C\limits^. {H_3}}\limits_{{\rm{Methyl free radicals}}} \to \mathop {C{H_3} - C{H_3}}\limits_{{\rm{Ethane}}} \)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionબેન્ઝિન અને તેના વ્યુત્પન્નો .... પ્રક્રિયા ખૂબ સરળતાથી દર્શાવે છે.
- 2ફ્રિડલ ક્રાફટસ પ્રક્રિયા નાં નિર્જળ $AlCl_3$ વપરાય છે, કારણ કે.....View Solution
- 3નીચેના પ્રક્રિયા ક્રમની અંતિમ નીપજ $C$ શું છે?View Solution
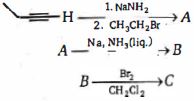
- 4View Solutionઆલ્કીન (પેરોક્સાઇડ્સની ગેરહાજરીમાં) સાથે હાઇડ્રોજન બ્રોમાઇડની પ્રતિક્રિયામાં, પ્રતિક્રિયાનું પ્રથમ તબબ્કો એ ............ છે
- 5નિર્જળ ઝિંક ક્લોરાઇડની હાજરીમાં $2-$મિથાઇલ પ્રોપીન સાથે એસિટાઇલ ક્લોરાઇડથી ગરમ કરતાં કાર્બનિક સંયોજન નિર્માણ પામે છે,તેનું સૂત્ર શું હશે?View Solution
- 6View Solutionબેન્ઝિનના ઓઝોનોલિસિસ (જળવિભાજન) થી મળતી નીપજ........
- 7કઈ પ્રક્રિયા દ્વારા $1$-બ્યુટીને એ બ્યુટેનમાં રૂપાંતર પામે છે ?View Solution
- 8View Solutionકઇ પ્રક્રિયામાં આલ્કેન અને આલ્કીન બંને મળે છે ?
- 9નીચેની પ્રક્રિયાઓના ક્રમમાં એક જ સમતલમાં $'C$'માં અણુઓની મહત્તમ સંખ્યા હાજર છેView Solution
$A \xrightarrow[ { Cu\; tube }]{\text { Redhot }}\mathrm{B} \xrightarrow[ Anhydrous AlCl_3]{\mathrm{CH}_{3} \mathrm{Cl}(1 \mathrm{eq}} \mathrm{C}$
($A$ એ સૌથી ઓછું પરમાણ્વીય વજન ધરાવતું આલ્કાઇન છે)
- 10View Solutionનીચેનામાંથી કયુ ચક્રીય સંયોજન કયુ છે?
