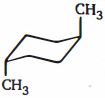
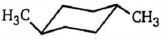
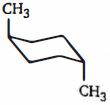
ટ્રાંસ- $ 1, 4 $ - ડાયમિથાઇલસાયકલોહેકઝેન નું સ્થાયી સ્વરૂપ શેના તરીકે રજૂ થાય છે
Diffcult
c
\((c)\) First . of all, there are two different dimethylcyclohexanes. One has both methyl substituents on the same side of the cyclohexane ring; it is the cis isomer (cis is Latin for "on this side"). The other has the two methyl substituents on opposite sides of the ring ; it is the trans isomer (trans is Latin for "across"). \(cis-1, 4\) -dimethylcyclohexane and \(trans -1, 4\) - dimethylcyclohexane are called geo"metric isomers of cis-trans stereoisomers - they have the same atoms, and the atoms are linked in the same order, but they differ in the spatial (stereo) arrangement of the atoms.
\((c)\) First . of all, there are two different dimethylcyclohexanes. One has both methyl substituents on the same side of the cyclohexane ring; it is the cis isomer (cis is Latin for "on this side"). The other has the two methyl substituents on opposite sides of the ring ; it is the trans isomer (trans is Latin for "across"). \(cis-1, 4\) -dimethylcyclohexane and \(trans -1, 4\) - dimethylcyclohexane are called geo"metric isomers of cis-trans stereoisomers - they have the same atoms, and the atoms are linked in the same order, but they differ in the spatial (stereo) arrangement of the atoms.
First we will determine which of the two chair conformers of \(cis- 1, 4\) - dimethylcyclohexane is more stable. One chair conformer has one methyl group in an equatorial position and one methyl group in an axial position. The other chair conformer also has one methyl group in an equatorial position and one methyl group in an axial position. Therefore, both chair conformers are equally stable.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$HO - \mathop {C{H_2}}\limits_{(2)} - \mathop {C{H_2}}\limits_{(3)} - F$View Solution
ઉપરના $C_2 - C_3$ સંયોજન કયો સુસંગત આજુબાજુ સૌથી સ્થાયી છે ?
- 2View Solutionનીચેનામાંથી કયો સંરૂપણ સમઘટકતા દર્શાવે છે ?
- 3$C_6H_{14}$ ના બંધારણીય સમઘટકોની સંખ્યા કેટલી છે ?View Solution
- 4View Solutionનીચેના સંયોજનનો નિરપેક્ષ વિન્યાસ ક્યો છે ?
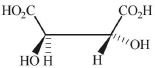
- 5નીચેનામાંથી કયું એ $2,3$ - બ્યુટેનડાયોલ નું મેસો બંધારણ નથી ?View Solution
- 6View Solutionસ્પીન સમઘટકતા કોણ બતાવે છે?
- 7$C{H_3}CH\left( {OH} \right)COOH$ દ્વારા દર્શાવાતી સમઘટકતા ...... છે.View Solution
- 8View Solutionનીચેનામાંથી ક્યા પ્રકાશક્યિાશીલ નથી ?
- 9View Solutionકયા બેના મિશ્રણ દ્વારા રેસેમિક મિશ્રણ બને છે ?
- 10View Solutionએસિટીક એસિડ અને મિથાઈલ ફોર્મેંટમાં કઈ સમઘટકતા દર્શાવે છે ?