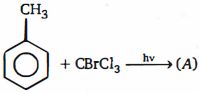
\((b)\) The use of light suggests a free radical mechanism. This means that the methane derivative will undergo homolytic fission. Since the \(C\) \(- Br\) bond is weaker than the \(C - Cl\) bond, it is reasonable to suppose that the former will be broken. Hence
\(\begin{gathered}
CBrC{l_3}\xrightarrow{{hv}}B{r^ \bullet }{ + ^ \bullet }CC{l_3} \hfill \\
PhC{H_3}\xrightarrow{{ \bullet CC{l_3}}}CHC{l_3} + PhC{H_2\bullet},\xrightarrow{{CBrC{l_3}}}PhC{H_2}Br + \,\,\bullet CC{l_3} \hfill \\
\end{gathered}\), etc
Attack by the free radical on toluene occurs at the methyl side chain and not in the ring because the \(C - H\) bond in \(Me\) is weaker than that of a ring- hydrogen atom and the benzyl free radical is far more stable than an aryl free radical.
The other point that requires explanation is why toluene is attacked by the \(CCl_3\) free radical and not by the bromine free radical. Activation energies involving free radicals are usually very low and so the controlling factor is the heat of reaction (or, more correctly, the free energy of reaction). The more exothermic the reaction (greater is \(\Delta G\)), the more favoured is that reaction. If the bromine atom attacks, the result is \(HBr\) , the bond of which is much weaker than the \(C - H\) bond formed when \(^\bullet CCl_3\) attacks. Hence, reaction
proceeds by the later route.
Download our appand get started for free
Similar Questions
- 1આ પ્રકિયા માં $Me -C \equiv C - Et \xrightarrow{{Na/liq.N{H_3}}}P\xrightarrow[{CC{l_4}}]{{B{r_2}}}(Q)$ ; $Q$ શું હશે ?View Solution
- 2View Solutionક્યા પ્રક્રિયકોની શ્રેણી દ્વારા એસિટિલિનનું ઉચ્ચ આલ્કાઇનમાં રૂપાંતર કરી શકાય છે ?
- 3View Solutionનીચેનામાંથી કયું સંયોજન સંસ્પંદન દર્શાવતું નથી?
- 4$n$-બ્યુટેનના ક્લોરિનેશન દ્વારા મળતા $C{H_3}\, - \,\,C{H_2}\, - \,\,\mathop C\limits_{\mathop |\limits_{Cl} } H\, - \,\,C{H_3}$ શું હશે ?View Solution
- 5View Solutionનીચેની પ્રક્રિયાની મુખ્ય નીપજ શોધો.
- 6View Solutionકેલ્શિયમ કાર્બાઇડની ભારે પાણી સાથેની પ્રક્રિયાથી શું મળે છે ?
- 7View Solutionનીચેના પૈકી કોની હેલોજન એસિડ સાથેની પ્રક્રિયા સૌથી ધીમા દરથી થશે ?
- 8નીપજ $(A)$ શું હશે ?View Solution
- 9View Solutionઆલ્કીન સામાન્ય રીતે કઈ પ્રકારની પ્રક્રિયા આપે છે?
- 10View Solutionનાઈટ્રેશન માટે સૌથી મહત્તમ પ્રક્રિયાશીલ કયું છે ?
