$1-$ બ્યુટિનને $2-$ બ્યુટિન દ્વારા ખૂબ સરળતાથી ઓળખી શકાય છે
AIIMS 2011, Diffcult
c
Tollen's reagent is ammonical silver nitrate which reacts with \(1-\) alkynes to form white percipitate of silver alkynide.
Tollen's reagent is ammonical silver nitrate which reacts with \(1-\) alkynes to form white percipitate of silver alkynide.
\(C{{H}_{3}}C{{H}_{2}}C\equiv CH+\underbrace{AgN{{O}_{3}}+N{{H}_{4}}OH}_{{}}\to \)
\(\underset{white\,ppt.}{\mathop{C{{H}_{3}}C{{H}_{2}}C\equiv CAg}}\,\downarrow +N{{H}_{4}}N{{O}_{3}}+{{H}_{2}}O\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1વોન્ટ હોફ પરિબળ $(i)$ શું હશે ?View Solution
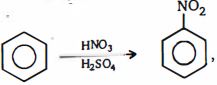
- 2View Solutionનીચેનામાંથી કયું હાઇડ્રોકાર્બન ઓરડાના તાપમાને પ્રવાહી અવસ્થામાં હશે?
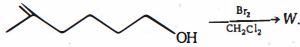
- 3નીપજ $W$ એ શું હશે ?View Solution
- 4View Solutionનીચેનામાંથી......એરોમેટીક છે ?
- 5$2$-બ્રોમોબ્યુટેનમાંથી હાઈડ્રોજન બ્રોમાઈડના વિલોપનના પરીણામે .......નું નિર્માણ થાય છે ?View Solution
- 6નીચેના પ્રક્રિયા ના ક્રમના નીપજ ની આગાહી કરોView Solution
ઇથાઇન $\xrightarrow[\begin{smallmatrix}
(2)\,\,excess\,I-\,C{{H}_{2}}\,-\,{{(C{{H}_{2}})}_{2}}-\,C{{H}_{3}} \\
(3)\,\,{{H}^{\oplus }}
\end{smallmatrix}]{(1)\,\,excess\,NaN{{H}_{2}}}$ - 7$U.V.$ પ્રકાશની હાજરીમાં બેન્ઝિનના બ્રોમીનેશનથી શું મળે છે ?View Solution
- 8નિપજો $P$ અને $Q$ નીચેની પ્રક્રિયાઓના કયા ક્રમમાં છે ?View Solution
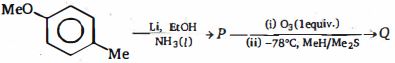
- 9View Solutionનીચેનામાંથી કયા સંયોજનમાંથી હાઇડ્રોજીનેશન પર પ્રકાશઅક્રિયાશીલ સંયોજન ઉત્પન્ન થાય છે
- 10View Solutionનીચેનામાંથી સૌથી વધારે બેઝિક સંયોજન છે...
