વોન્ટ હોફ પરિબળ $(i)$ શું હશે ?

Medium
c
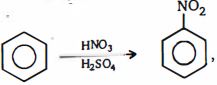
$(c)$ This is borne out by the fact that solutions of nitric acid in pure sulphuric acid show an almost four-fold molecular freezing-point depression (actual ly $i \approx 3.82$), which has been interpreted as being due to formation of the four ions
$(c)$ This is borne out by the fact that solutions of nitric acid in pure sulphuric acid show an almost four-fold molecular freezing-point depression (actual ly $i \approx 3.82$), which has been interpreted as being due to formation of the four ions
$(1)$ $H\ddot O\, - \,N{O_2}\,\overset {{H_2}S{O_4}} \longleftrightarrow {H_2}\mathop O\limits_ \oplus - N{O_2}$ $\overset {{H_2}S{O_4}} \longleftrightarrow {H_2}O{ - ^ \oplus }N{O_2}$
$(2)$ ${H_2}O\, + \,{H_2}S{O_4}\, \rightleftharpoons \,{H_3}{O^ \oplus }\, + \,HSO_4^\Theta $
net reaction, i.e., $HN{O_3}\, + \,2{H_2}S{O_4}\, \rightleftharpoons $ $^ \oplus N{O_2}\, + {H_3}{O^ \oplus } + \,2HSO_4^\Theta $
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1બેન્ઝિનની $C - C$ બંધલંબાઇ એ...........View Solution
- 2જ્યારે ઓક્સિડેટીવ ઓઝોનોલિસિસમાંથી પસાર થાય છે ત્યારે કયું સંયોજન એ $CO_2$ ગેસ મુક્ત કરતો નથી ?View Solution
- 3View Solutionબેયરનો પ્રક્રિયક ....
- 4View Solutionનીચેના પૈકી આકારની ર્દષ્ટિએ સમતલીય સંયોજન કયું છે ?
- 5$3, 3-$ ડાયમિથાઇલ $-2-$બ્યુટેનોલને ${H_2}S{O_4}$ સાથે ગરમ કરતા મળતી મુખ્ય નીપજ કઇ હશે ?View Solution
- 6$\left( C _{7} H _{5} O _{2}\right)_{2} \xrightarrow{hv} [ X ]+2 \dot{ C }_{6} H _{5}+2 CO _{2}$View Solution
ઉપરની પ્રક્રિયાને ધ્યાનમાં લો. અને મધ્યવર્તી ' $X$ ' ને ઓળખો.
- 7View Solutionકેલ્શિયમ કાર્બાઇડની પાણી સાથેની પ્રક્રિયાથી....... મળે છે.
- 8પદાર્થ $'A'$ પર ક્લોરીનેશનથી પદાર્થ $'B'$ આપે છે. પદાર્થ $'B'$ ને આલ્કોહોલીક $KOH$ સાથે પ્રક્રિયા કરતા પદાર્થ $'C'$ આપે છે. કે જે બેયરના પ્રક્રીયકને રંગવિહીન કરે છે. પદાર્થ $ 'C'$ નું ઓઝૉનોલીસીસથી $HCHO$ આપે છે પદાર્થ $ 'A'$ શું હશે ?View Solution
- 9View Solutionફ્રિડલ-ક્રાફટ્સ પ્રક્રિયામાં કયા પ્રક્રિયકો વાપરી શકતા નથી ?
- 10$RCH = CH_2$ ને સાથે $B_2H_6$ સાથે આલ્કલાઈન $H_2O_2$ સાથે ઓક્સિડેશન કરતાં શું મળે છે ?View Solution
