$1-$મિથોક્સી$-1, 3-$બ્યુટાડાઇનના નીચેનામાંથી સંસ્પંદનીય રચનાઓમાંથી કયું ઓછામાં ઓછું સ્થાયી છે?
IIT 2005, Diffcult
c
(c) The octet of all atoms are complete in structures \((a)\) and \((b)\). In structure \((d)\) electron deficiency of positively charged carbon is duly compensated by lone pair electrons of adjacent oxygen atom while such neighbour group support is not available in structure \((c)\).
(c) The octet of all atoms are complete in structures \((a)\) and \((b)\). In structure \((d)\) electron deficiency of positively charged carbon is duly compensated by lone pair electrons of adjacent oxygen atom while such neighbour group support is not available in structure \((c)\).
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીયે આપેલા સંયોજનો માટે ઈલેકટ્રોન અનુરાગી વિસ્થાપન તરફ સક્રિયતાનો ઉતરતો ક્રમ શોધો.
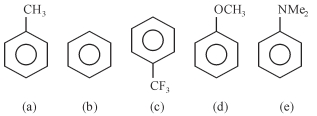
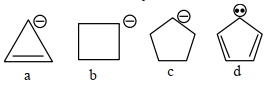
- 2View Solutionકાર્બએનાયન ની સ્થિરતા નો સાચો ક્રમ શોધો.
- 3આપેલ કાર્બોકેટાયન માટે સ્થિરતાનો સાચો કમ કયો છે ?$\mathop {\mathop C\limits^ \oplus {H_3}}\limits_{(i)} \,\,\,\,\,\,\,\,\,C{H_3}\mathop {\mathop C\limits^ \oplus {H_2}}\limits_{(ii)} \,\,\,\,\,\,\,\,\mathop {\mathop C\limits^ \oplus {H_2}}\limits_{(iii)} OC{H_3}\,$View Solution
- 4નીચેના આલ્કેન્સ સમ વિભાજન દ્વારા મુક્ત મુલક બનાવે છે.View Solution
$CH_3 - CH_3, CH_3 - CH_2 $$- CH_3, (CH_3)_2CH - CH_3,$
$ CH_3 - CH_2 - CH(CH_3)_2.$
આ મૂલકોનો સ્થાયિતાનો વધતો ક્રમ કયો છે?
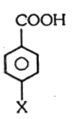
- 5આ પદાર્થમાં $ X$ તરીકે ...... હોય તો પદાર્થના $ pKa$ મૂલ્ય ઘટાડો થાય છે.View Solution
- 6View Solutionબેઝિકતાનો સાચો ક્રમ કયો છે ?
- 7View Solutionનીચે આપેલા પૈકી એરોમેટિક ઈલેકટ્રોનઅનુરાગી વિસ્થાપન પ્રક્રિયામાં અક્રિયકારક સમૂહોની કુલ સંખ્યા__________ છે.
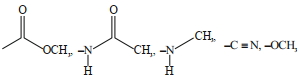
- 8View Solutionપ્રક્રિયાની ક્રિયાવિધિ માં ઇલેક્ટ્રોનની ગતિ બતાવવા માટે કાર્બનિક રસાયણશાસ્ત્રમાં વક્ર તીરનો ઉપયોગ કરવામાં આવે છે. નીચેની પ્રક્રિયાની યોગ્ય નીપજ કઈ છે
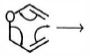
- 9View Solutionઝાયલેન્સમાં, જે ઉષ્માગતિકીયરીતે સૌથી સ્થાયી કયું છે?
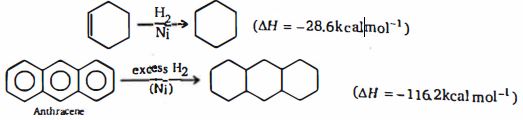
- 10નીચે આપેલા સવાલના જવાબ માટે નીચે આપેલી માહિતી નો ઉપયોગ કરો.View Solution
એન્થ્રેસીનની સન્સ્પંદીય ઉ ર્જાની ગણતરી કરો .......$kcal/mol$