$2\; \mathrm{M}$ જલીય $\mathrm{NaOH}$ના દ્રાવણની ઘનતા $1.28 \;\mathrm{g} / \mathrm{cm}^{3} $ છે, આ દ્રાવણની મોલાલિટી .......$m$ છે.
[અહીં $\mathrm{NaOH}$નું આણ્વિય દળ $=40 \;\mathrm{g} \mathrm{mol}^{-1}$]
NEET 2019, Medium
c
\(2 \mathrm{M}\) solution of \(\mathrm{NaOH}\) means 2 mole \(\mathrm{NaOH}\) is present in \(1 \mathrm{L}\) solution; density \(=1.28 \mathrm{g} / \mathrm{ml}\)
\(2 \mathrm{M}\) solution of \(\mathrm{NaOH}\) means 2 mole \(\mathrm{NaOH}\) is present in \(1 \mathrm{L}\) solution; density \(=1.28 \mathrm{g} / \mathrm{ml}\)
mass of solution \(=\) volume of solution \(\times\) density \(=1000 \times 1.28\)
\(=1280 \mathrm{g}\)
mass of solvent \(=\) mass of solution \(-\) mass of solute \(=1280-80\)
\(=1200 \mathrm{g}\)
molality \(=\frac{2}{1200} \times 1000=\frac{20}{12}=\frac{10}{6}=\frac{5}{3}=1.67 \mathrm{m}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1આયનના સ્ફીટકમય પદાર્થનું પ્રમાણ સૂચક સૂત્ર $Fe_2(SO_4)_3$ છે. તે પાણીમાં વપરાય છે અને સુએજ ટ્રીટમેન્ટમાં આલંબિત અશુધ્ધીઓ દુર કરવા માટે વપરાય છે. આ પદાર્થમાં આયર્ન, સલ્ફર અને ઓક્સિજનની ટકાવારી અનુક્રમે......છે.View Solution
- 2$MCl_3$, $4H_2O$ સૂત્ર ધરાવતા સંકિર્ણ સંયોજનમાં ધાતુનો સવર્ગ આંક $6$ છે. તથા કોઈ પણ અણુ જલીયકરણ ધરાવતું નથી. તો આ સંયોજનનું $200 \,ml$ $0.01\, M$ દ્રાવણમાંથી ક્લોરિન આયનોને છુટા પાડવા માટે $0.1\, M$ $AgNO_3$ નું કેટલા .............. $\mathrm{ml}$ કદ જરૂરી છે ?View Solution
- 3$100\, mL \,2 \,M\, KCl$ અને $200\, mL \,3 \,M\,K_2SO_4$ ના દ્રાવણોને મિશ્ર કરતા મિશ્રણમાં $K^+$ આયનની મોલારિટી કેટલા .......... $\mathrm{M}$ થશે ?View Solution
- 4જો ઘઉંના $4$ દાણાને ગણવા માટે એક સેકન્ડ લાગે તો, એક મોલ ઘઉંના દાણાની ગણતરી કરવા માટે લાગતો સમય ......વર્ષ.જView Solution
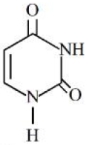
- 5નીચે આપેલ બંધારણ સાથે $RNA$ માં હાજર યુરેસીલ બેઇઝ છે. યુરિસિલમાં $N$ ના $\%............$ છે.View Solution
મોલર દળ $N =14\,g\,mol ^{-1} ; O =16\,g\,mol ^{-1} ; C =12\,g\,mol ^{-1} ; H =1\,g\,mol ^{-1}$;
- 6$20\,mL$ કેલ્શિયમ હાઈડ્રોકસાઈડ વપરાય છે જ્યારે તેને તેની પ્રક્રિયા $10\,mL\,H _2 SO _4$ ના અજ્ઞાત દ્રાવણ સાથે પ્રક્રિયા કરવામાં આવે છે.આ ઉપરાંત ફિનોલ્ફથેલીનના $2$ ટીપાંઓ ધરાવતા $0.5\,M\,HCl$ ના $20\,mL$ પ્રમાણિત દ્રાવણનું કેલ્શિમ હાઇડ્રોકસાઈડ સાથે અનુમાપન કરવામાં આવે છે.મિશ્રણ ગુલાબી રંગ દર્શાવે છે જ્યારે બ્યુરેટ $35.5\,mL$ નું મૂલ્ય દર્શાવ્યું હતું જ્યાં બ્યુરેટ શરૂઆતમાં (પ્રારંભમાં) $25.5\,mL$ દર્શાવ્યું હતું.તો $H _2 SO _4$ ની સાંદ્રતા $......\,M$ છે.(નજીકનો પૂર્ણાક)View Solution
- 7એક દ્વિ- સંયોજક ધાતુનો તુલ્યભાર $32$ છે, તો તે ધાતુના નાઇટ્રેટ ક્ષારનો દળ કેટલો હશે?View Solution
- 8સલ્ફરનો ઓક્સાઈડ એ $50\%$ સલ્ફર ધરાવે છે. તેનું પ્રમાણસુચક સૂત્ર કયુ હશે ?View Solution
- 9પાણીમાં $15$ ગ્રામ $NaOH$ ને દ્રાવ્ય કરતા $250$ મિલી દ્રાવણ બની શકાય છે. આ દ્રાવણની મોલારીટી......$M$ માં શોધો.View Solution
- 10$0.205$ મોલ $Ba(OH)_2$ માંથી વધુ પ્રમાણમાં $CO_2$ પસાર કરતાં કેટલા .............. ગ્રામ $BaCO_3$ ઉત્પન્ન થાય ?View Solution
