$300 K$ તાપમાને રહેલ પાત્રમાં એક મોલ ઓક્સિજન અને બે મોલ નાઇટ્રોજન વાયુ ભરેલ છે.${O_2}$ની સરેરાશ ચાકગતિઉર્જા અને ${N_2}$ ની સરેરાશ ચાકગતિઉર્જાનો ગુણોત્તર કેટલો થાય?
IIT 1998, Medium
a
A diatomic gas has \(2\) degrees of freedom associated with rotational motion. Law of equipartition of energy states that the rotational kinetic energy per degree of freedom is \(\frac{1}{2} KT\). Since two gases are at same temperature their rotational kinetic energies will be equal.
A diatomic gas has \(2\) degrees of freedom associated with rotational motion. Law of equipartition of energy states that the rotational kinetic energy per degree of freedom is \(\frac{1}{2} KT\). Since two gases are at same temperature their rotational kinetic energies will be equal.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$CO_2 (O - C - O)$ એ ત્રિઆણ્વિય વાયુ છે. વાયુના $1\,gm$ ની સરેરાશ ગતિ ઊર્જા ......છે. $N$એ એવોગેડ્રો અંક, $k $- બોલ્ટઝમેન અચળાંક અને $CO_2$ નો અણુભાર $=44$View Solution
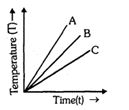
- 2$A, B$ અથવા $C$ માંથી કયા પદાર્થની વિશિષ્ટ ઉષ્મા સૌથી વધુ છે? તાપમાન સમયનો આલેખ દર્શાવેલો છે?View Solution
- 3$ {O_2} $ ની $rms$ ઝડપ $400\, m/sec$ છે,તો તે તાપમાને $ {H_2} $ ની $rms$ ઝડપ ..... $m/sec$ હશે?View Solution
- 4અચળ દબાણ થર્મોમીટર માં થર્મોમીટર જ્યારે તે બરફ જેવા પાણીમાં ડુબાડેલ હોય ત્યારે $47.5$ એકમ કદ માપે અને જ્યારે ઉબળતા પ્રવાહીમાં રાખવામા આવે ત્યારે તે $67$ એકમ કદ માપે છે.તો પ્રવાહીનું ઉત્કલનબિંદુ .......... $^oC$ હશે?View Solution
- 5બે મોલ એક પરમાણ્વિક આદર્શ વાયુને બંધ પાત્રમાં રાખી તેને ગરમ કરતાં તેનું તાપમાન $10\,^oC$ વધે છે.તો તેની આંતરિક ઉર્જામાં થતો ફેરફાર ..... $J$ હશે. $(R = 8.31\, J/mole-K)$View Solution
- 6$27^{\circ} C$ તાપમાને અને $1$ વાતાવરણ દબાણે રહેલા વાયુ માટે આપેલા દળ ધરાવતા અણુઓની સરેરાશ વર્ગિત વર્ગમૂળ $(r.m.s.)$ ઝડપ $200\, ms ^{-1}$ છે. $127^{\circ} C$ તાપમાને અને $2$ વાતાવરણ દબાણે રહેલા વાયુ માટે અણુઓની સરેરાશ વર્ગિત વર્ગમૂળ ઝડપ $\frac{ x }{\sqrt{3}}\, ms ^{-1}$ છે. $x$ નું મૂલ્ય ......... $ms ^{-1}$ હશે.View Solution
- 7અચળ દબાણે, આદર્શ વાયુના કદમાં પ્રતિ કેલ્વિન તાપમાનના વઘારાએ થતો વઘારો અને વાયુના મૂળ કદનો ગુણોત્તર ... છે. (જયાં $T$ = વાયુનું નિરપેક્ષ તાપમાન છે.)View Solution
- 8ચોક્કસ વાયુના અણુઓનો $STP$ એ સરેરાશ મુક્ત પથ $1500\,d$ છે, જ્યાં $d$ એ વાયુના અણુઓનો વ્યાસ છે. પ્રમાણભૂત દબાણ જાળવી રાખતા, $ 373\,K$ પર અંદાજિત સરેરાશ મુક્ત પથ સરેરાશ ........... $d$ છે.View Solution
- 9વાન્ડર વાલ્સ વાયુ માટે જો $P_c, V_c$ અને $T_c$ અનુક્રમે ક્રિટિકલ દબાણ, કદ અને તાપમાન હોય તો $P_cV_c/T_c$ ......View Solution
- 10View Solutionરસોઇ કરવા સમાન્ય ધાતુનું વાસણ ઘણું અનુકૂળ છે કારણ કે ......
