$4 \,Nm^{-2}$ ના અચળ બાહ્ય દબાણે, એક આદર્શ વાયુ $5\, m^3$ થી $1\, m^3$ સુધી સમતાપી સંકોચન પામે છે. આ પ્રક્રિયામાં ઉત્સર્જીત ઊષ્માનો ઊપયોગ $1$ મોલ $Al$ ને ગરમ કરવા માટે આવે છે. જો $Al$ ની મોલર ઊષ્મા ક્ષમતા $24\, J\, mol^{-1}K^{-1}$ હોય તો, $Al$ નું તાપમાન કેટલું વધશે?
JEE MAIN 2019, Medium
c
\(q = P\Delta V\)
\(q = P\Delta V\)
\(q = 16\)
\({C_P} = 24\)
\({C_P} = \frac{{qP}}{{\Delta T}}\)
\(\Delta T = \frac{{16}}{{24}}\,K = \frac{2}{3}\,K\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1અચળ $T$ અને $P$ એ $CO(g) + \frac{1}{2}{O_2}(g) \to C{O_2}(g)$ પ્રકિયા માટે નીચેનામાથી કયું વિધાન સાચું છે ?View Solution
- 2સમતાપી પરિસ્થિતિમાં $300 \;\mathrm{K}$ પર તેમજ $10^{5}\; \mathrm{Nm}^{-2} $ ના અચળ દબાણના લીધે એક વાયુ $10^{-3} \;\mathrm{m}^{3}$ માંથી $10^{-2} \;\mathrm{m}^{3}$માં વિસ્તરણ પામે છે. તો વાયુ વડે થતુ કાર્ય શું હશે ?View Solution
- 3પ્રક્રિયા માટે $ℓ\,n\, K_{eq}$ વિરૂદ્ધ તાપમાનનો આલેખ દોરવામાં આવે તો પ્રક્રિયા...... હોવી જોઈએ.View Solution
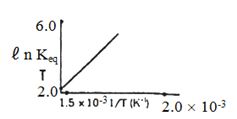
- 4View Solutionસમોષ્મી વિસ્તરણ કરતા સમતાપી પ્રકમમાં વાયુના દબાણના ઘટાડા સાથે કદનો વધારો વધારે હોય છે, કારણ કે .............
- 5View Solutionપ્રક્રિયા માટે એન્થાલ્પીનો ફેરફાર ........ પર આધારિત નથી.
- 6View Solutionઅવસ્થા વિધેય .......
- 7View Solutionકોઈ પણ પ્રબળ એસિડ તેમજ પ્રબળ બેઈઝની તટસ્થીકરણ એન્થાલ્પીનું મૂલ્ય અચળ હોય છે, કારણકે......
- 8View Solutionસ્વયંભુ પ્રક્રિયાને લાગુ પડતું સાચુ વિધાન ઓળખો.
- 9$-33.42^{\circ}\,C$ અને $1\,bar$ દબાણ પર $NH _{3}$ ના $17.0\,g$ સંપૂર્ણ બાષ્પીકરણ પામે છે અને આ પ્રક્રમમાં એન્થાલ્પી ફેરફાર $23.4\,kJ\,mol ^{-1}$ છે. સમાન પરિસ્થિતિઓ હેઠળ $85 g\,NH _{3}$ ના બાષ્પીકરણ માટે એન્થાલ્પી ફેરફાર $\dots\dots\dots\,\,kJ$ છે.View Solution
- 10$298 \,K$ તાપમાને $C - H, C - C, C = C$ અને $H - H$ ની બંધ-ઊર્જા અનુક્રમે $414, 347, 615$ અને $435$ કિલો જૂલ મોલ$^{-1}$ છે, તો ${H_2}C = C{H_{2(g)}} + {H_{2(g)}} \to {H_3}C - C{H_{3(g)}}$પ્રક્રિયા દરમિયાન $298\, K$ તાપમાને એન્થાલ્પી-ફેરફાર કેટલા .....કિલો જૂલ થશે ?View Solution
