આયનીય સંયોજનમાં $A^+ X^-$ સહસંયોજક બંધનનો અંશ શ્રેષ્ઠ છે જ્યારે
Easy
b
Due to Fazan's rule, according to which the cation distorts the electron cloud of the anion and pulls the electron density towards itself. Thus it introduces some covalent character in the lonic bond. Now, the smaller the cation, greater is its polarising power and larger the anion, greater will be its polarisability hence \(A +\) is small and \(X -\) is large.
Due to Fazan's rule, according to which the cation distorts the electron cloud of the anion and pulls the electron density towards itself. Thus it introduces some covalent character in the lonic bond. Now, the smaller the cation, greater is its polarising power and larger the anion, greater will be its polarisability hence \(A +\) is small and \(X -\) is large.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1આયનીય સંયોજનમાં $A^+ X^-$ સહસંયોજક બંધનનો અંશ શ્રેષ્ઠ છે જ્યારેView Solution
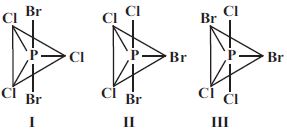
- 2$PCl_3Br_2$ ભૌમિતિક સમઘટકતા દર્શાવે છે, જેના ભૌમિતિક સમઘટકો નીચે મુજબ છે. તે પૈકી કોણ દ્વિધ્રુવ ચાકમાત્રા ધરાવતો(તા) નથી ?View Solution
- 3View Solutionનીચેનાં પૈકી સૌથી ટૂકો બંધ ધરાવતો ઘટક જણાવો.
- 4એસાયક્લીક $C _4 H _5 N$ માં $sp ^3$ સંકરીત કાર્બનની સંખ્યા $.....$ છે.View Solution
- 5View Solutionઆણ્વીય કક્ષક સિદ્ધાંત અનુસાર નીચેનામાંથી કોનું અસ્તીત્વ નથી?
- 6View Solutionઆયનિક ઘન વિદ્યુતનો મંદ વાહક હોય છે તેનું કારણ .......
- 7$O_{2}^{2-}$ના તમામ બંધનીય આણ્વિય કક્ષકમાં ઇલેક્ટ્રોનની કુલ સંખ્યા $......$ છે.View Solution
- 8View Solutionનીચે પૈકી કયું સૌથી વધારે દ્વિધ્રુવીય ચાકમાત્રા ધરાવે છે?
- 9View Solutionનીચે આપેલી સ્પીસીઝની જોડીઓમાંથી કઈ સમ-બંધારણીય નથી ?
- 10$N_2$ અણુ ના પરમાણુમાં કેટલા બંધ હોય છેView Solution
