અચળ દબાણ $100\, N/m^2$ એ વાયુનું કદ $2\,m^3$ થી $1\,m^3$ થાય છે.પછી તેને અચળ કદે ગરમ કરવા $150\, J$ ઉષ્મા આપવામાં આવે છે. વાયુની આંતરિક ઉર્જામાં કેટલો ફેરફાર થાય?
JEE MAIN 2014, Medium
a
As we know, \(\Delta Q=\Delta u+\Delta w\)
As we know, \(\Delta Q=\Delta u+\Delta w\)
(Ist law of thermodynamics) \(\Rightarrow \Delta \mathrm{Q}=\Delta \mathrm{u}+\mathrm{P} \Delta \mathrm{v}\)
or \(150=\Delta u+100(1-2)\)
\(=\Delta u-100\)
\(\therefore \Delta u=150+100=250 \mathrm{J}\)
Thus the internal energy of the gas increases by \(250 \mathrm{J}\)
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1$2$ મોલ એક પરમાણ્વિક વાયુનું તાપમાન $273 K$ થી $373 K$ કરવા માટે જરૂરી ઉષ્માઊર્જા ...... $R$ થાય? પ્રક્રિયામાં કાર્ય થતું નથી.View Solution
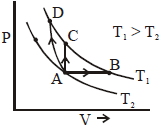
- 2એક પરમાણ્વિક આદર્શ વાયુ માટેની ત્રણ અલગ-અલગ પ્રક્રિયા માટે $P$ વિરુધ્ધ $V$ નો ગ્રાફ આપેલ છે. તેમના પથને $A \rightarrow B, A \rightarrow C$ અને $A \rightarrow D .$ વડે દર્શાવેલ છે. આ પ્રક્રિયા દરમિયાન થતો આંતરિક ઉર્જાનો ફેરફાર $E _{ AB }, E _{ AC }$ અને $E _{ AD }$ અને થતાં કાર્યને $W _{ AB }$ $W _{ AC }$ અને $W _{ AD }$ વડે દર્શાવેલ છે. તો આપેલ પરિણામો વચ્ચે સાચો સંબંધ શું થશે?View Solution
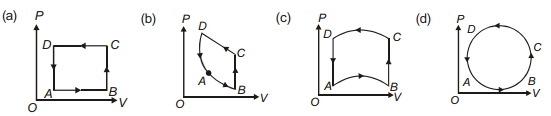
- 3નીચેની આકૃતિઓ $(a)$ થી $(b)$ માં દબાણની ફેરફાર વડે કદમાં થતો ફેરફાર આકૃતિમાં દશાવેલ છે. વાયુને પથ $A B C D A$ પર લાવવામાં આવે છે. વાયુની આંતરિક ઉર્જામાં થતો ફેરફાર .......... છે.View Solution
- 4કોઈ એક પ્રક્યિામાં એક મોલ એક પરમાણ્વીક આદર્શ વાયુના કદ અને તાપમાનમાં $ VT=K$ ના સબંધ અનુસાર બદલાય છે. જ્યાં $K$ એ અચળાંક છે. આ પ્રક્રિયામાં વાયુના તાપમાનને $\Delta T$ જેટલું વધારવામાં આવે છે. વાયુ દ્વારા શોષાતી ઊષ્માનો જથ્થો કેટલો હશે. ($R$ વાયુ અચળાંક છે).View Solution
- 5$2$ મોલ એક પરમાણ્વિક વાયુનું તાપમાન $273 K$ થી $373 K$ કરવા માટે જરૂરી ઉષ્માઊર્જા ...... $R$ થાય? પ્રક્રિયામાં કાર્ય થતું નથી.View Solution
- 6કાર્નોટ એન્જિન $ {411^o}C $ અને $ {69^o}C $ વચ્ચે કાર્ય કરે છે.તેને ચક્રદીઠ અપાતી ઉષ્મા $1000 \,J$ હોય,તો ચક્ર દીઠ ....... $J$ કાર્ય થશે?View Solution
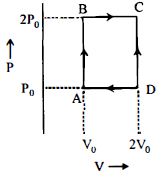
- 7$n$ મોલ આદર્શ વાયુ એન્જિન $ABCD$ પથ પર ચક્રિય પ્રક્રિયા કરે છે તો એન્જિનની ઉષ્મા કાર્યક્ષમતા કેટલી થાય? ($C_v =1 .5\, R$, $R-$ વાયુ અચળાંક)View Solution
- 8એક આદર્શ ઉષ્મીય યંત્ર માટે, સ્ત્રોતનું તાપમાન $127\,^{\circ} C$ છે. $60\, \%$ જેટલી કાર્યક્ષમતા મેળવવા માટે, ઠારણનું તાપમાન $........\,{ }^{\circ} C$ હોવું જોઈએ. (નજીકત્તમ પૂર્ણાકમાં લખો)View Solution
- 9View Solutionનીચે આપેલા કયા તાપમાન માટે કાર્નોટ એન્જિનની કાર્યક્ષમતા મહત્તમ હોય.
- 10કાર્નોટ એન્જિનની કાર્યક્ષમતા $50\%$ હોય,જયારે ઠારણ વ્યવસ્થાનું તાપમાન $7 °C$ હોય છે.કાર્યક્ષમતા $70\%$ કરવા માટે ઉષ્મા પ્રાપ્તિસ્થાનનું તાપમાન ...... $K$ વધારવું પડે?View Solution
