$\mathop {{H_3}C\mathop C\limits^ \ominus {H_2}}\limits_{(i)} \,,$$\mathop {{H_2}C = \mathop C\limits^ \ominus H}\limits_{(ii)} $ અને $\mathop {H - C \equiv \mathop C\limits^ \ominus }\limits_{(iii)} $
Weaker the acid, strongest is its conjugate base.
Among alkane, alkene and alkyne are most acidic and alkanes are least acidic, so the order of base strengths
alkane \(>\) alkene \(>\) alkyne
\(C H_{3} \stackrel{\ominus}{C} H_{2}>H_{2} C=\stackrel{\ominus}{C} H>H-C \equiv {C}^{\ominus}\)
Download our appand get started for free
Similar Questions
- 1View Solutionકોણ સૌથી વધુ સ્થિર છે ?
- 2View Solutionનીચેના પદાર્થો માટે વધતી એસિડિકતાનો સાચો ક્રમ કયો છે ?
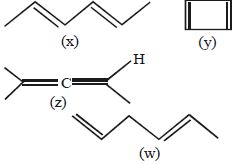
- 3View Solutionનીચેનામાંથી કયો સૌથી સ્થિર કાર્બોકેટાયન છે ?
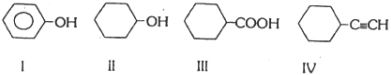
- 4View Solutionનીચેનામાંથી કયો સૌથી એસિડિક પદાર્થ છે ?
- 5View Solutionકયો પદાર્થ ન્યુનતમ હાઈડ્રોજીનેશન ઉષ્મા ધરાવે છે ?
- 6નીચેનાને તેમના $pK_a$ મૂલ્યોના વધતા ક્રમમાં ગોઠવોView Solution
$(x)\begin{array}{*{20}{c}}
{O\,\,\,}\\
{||\,\,\,}\\
{C{H_3} - S - O - H}\\
{||\,\,\,\,}\\
{O\,\,\,\,}
\end{array}$$\begin{array}{*{20}{c}}
{\,\,\,\,\,O}\\
{\,\,\,\,\,\,||}\\
{(y)\,\,\,C{H_3} - C - O - H}
\end{array}$$(z)\,\, CH_3 -OH$
- 7View Solutionએસિડિક સ્વભાવનો ક્રમ શું હશે ?
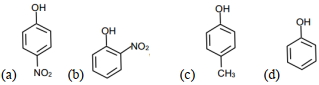
- 8નીચેના ઘટકો $(A)$, $(B)$,અને $(C)$ $(D)$માંથી કયા ઘટકો $sp^2$ સંકૃત કાર્બન ધરાવે છે ?$CH_3^ + \,,\,\,\,CH_3^ - \,\,,\,\,\mathop C\limits^ \bullet {H_3}\,,\,\,C{H_2}$View Solution
- 9નીચેના કાર્બોકેટાયનની સ્થાયિતામાં ઘટાડો થવાનો સાચો ક્રમ કયો છેView Solution
$\mathop {C{H_3} - \mathop C\limits^ \oplus H - C{H_3}}\limits_I $
$\mathop {C{H_3} - \mathop C\limits^ \oplus H - OC{H_3}}\limits_{II} $
$\mathop {C{H_3} - \mathop C\limits^ \oplus H - C{H_2} - OC{H_3}}\limits_{III} $
- 10View Solutionનીચેના ઘટકોનો સ્થાયિતાનો સાચો ક્રમ જણાવો;