એસિડિક સ્વભાવનો ક્રમ શું હશે ?

AIIMS 2019, Medium
b
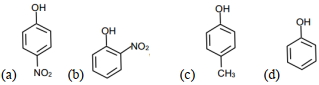
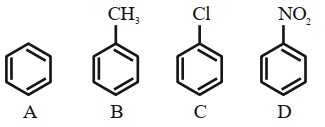
In compound \((a)\), \(NO _{2}\) possesses the \(- M\) effect at para position due to which it is highly acidic than other three compounds. The compound \((b)\) has intermolecular hydrogen bonding and it is less acidic than \((a)\). In compound \((d)\), there is absence of an electron donating group, due to which it is more acidic than compound \((c)\), which has electron donating methyl group. Therefore, the correct order of acidic nature is \(a > b > d > c\)
In compound \((a)\), \(NO _{2}\) possesses the \(- M\) effect at para position due to which it is highly acidic than other three compounds. The compound \((b)\) has intermolecular hydrogen bonding and it is less acidic than \((a)\). In compound \((d)\), there is absence of an electron donating group, due to which it is more acidic than compound \((c)\), which has electron donating methyl group. Therefore, the correct order of acidic nature is \(a > b > d > c\)

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1પીક્રીક એસિડ, એસિટિક એસિડ અને ફિનોલના $ H_2O$ માં $ pK_a $ મૂલ્ય કયા ક્રમમાં છેView Solution
- 2View Solutionકઈ જોડીમાં બીજું આયન પ્રથમ કરતા વધુ સ્થાયી છે?
- 3View Solutionકયો પદાર્થ સૌથી નીચો ડાયપોલ મોમેન્ટ ધરાવે છે ?
- 4View Solutionનીચેનામાંથી કયો જલીય માધ્યમમાં પ્રબળ બેઈઝ છે ?
- 5$ E.A.S. $ સ્થાન લેશે તે સ્થાન ઓળખોView Solution
- 6જે સ્થાયીતાનો ઘટતો ક્રમ છેView Solution
$(i)$ $C{H_3} - \mathop C\limits^ + H - C{H_3}$
$(ii)$ $C{H_3} - \mathop C\limits^ + H - O - C{H_3}$
$(iii)$ $C{H_3} - \mathop C\limits^ + H - CO - C{H_3}$
- 7View Solutionનીચેનામાંથી કયો જલીય માધ્યમમાં પ્રબળ બેઈઝ છે ?
- 8View Solutionઈલેકટ્રોન અનુરાગી વિસ્થાપન પ્રક્રિયા પ્રત્યેની સક્રિયતાનો ક્રમ જણાવો.
- 9View Solutionનીચે પૈકી કોની દ્વિધ્રુવીય ચાકમાત્રા સૌથી વધારે છે?
- 10View Solutionઆપેલ ધનાયનમાં, સૌથી સ્થાયી કાર્બોનિયમ આયન કયો છે?
