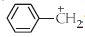
All carbocations are $sp^2$ hybridised and they have planar triangular structure. The value of the $\mathrm{H}=3$ only.
$\mathrm{H}=\frac 12[$ Valence electron of the central atom $+$ no of surrounding atomcation $+$ anion]
$=\frac 12[4+3-1+0]$
$=6 / 2=3$
Download our appand get started for free
Similar Questions
- 1નીચે બતાવેલ પરમાણુઓમાંથી કયા રેખાંકિત પરમાણુ $sp-$ સંકરણ છે ?View Solution
$\begin{array}{*{20}{l}}
{(u){H_2}\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle-}$}}{C} CHC{H_3}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} (v){\mkern 1mu} C{H_2}\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle-}$}}{C} CHCl{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} (w){\mkern 1mu} C{H_3}\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle-}$}}{C} H_2^ + {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} (x){\mkern 1mu} H - C \equiv C - H} \\
{(y){\mkern 1mu} C{H_3}\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle-}$}}{C} N{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} (z){\mkern 1mu} {{(C{H_3})}_2}C\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle-}$}}{N} N{H_2}}
\end{array}$ - 2સાયકલોબ્યુટાઇલડાયએનાઇલ ઋણાયન ${({C_4}{H_4})^{ - 2}}$માં $\pi $ ઇલેક્ટ્રોનની સંખ્યા કેટલી છે?View Solution
- 3આપેલ સંયોજન $CH\equiv C-CH=CH_2$નું $IUPAC$ નામ શું છેView Solution
- 4સયોજન $C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{\underset{C{{H}_{2}}}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,}}\,}}\,-C{{H}_{2}}-CH(OH)-C{{H}_{3}}$ નું $\,\text{IUPAC }$ નામ .........છેView Solution
- 5સંયોજનનું $IUPAC$ નામ શું છે?View Solution
$\begin{array}{*{20}{c}}
{C{H_3} - C = CH - C{H_2} - COOH} \\
{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,} \\
{OH\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}
\end{array}$ - 6પદાર્થનું $ IUPAC $ નામ જણાવો. $ (CH_3)_3C - CH = CH_2$View Solution
- 7આ $CH_2 = CH -CH (CH_3)_2$ સંયોજન નું $IUPAC$ શું હશેView Solution
- 8$\begin{matrix}View Solution
C{{H}_{2}}-CN \\
|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
C{{H}_{2}}-CN \\
\end{matrix}$ નું $IUPAC$ નામ શું હશે ? - 9આપેલ પદાર્થનું $IUPAC $ નામ જણાવો $.(CH_3)_2C(CH_2CH_3)CH_2CH(Cl)CH_3$View Solution
- 10નીચે પૈકી કયું સંયોજન જે કાર્બન બંધ બનાવવા માટે માત્ર $s{p^3}$ સંકૃત કક્ષકનો ઉપયોગ કરે છે.View Solution
