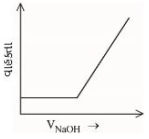
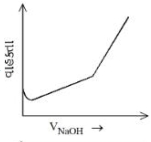
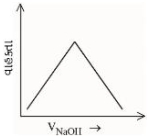
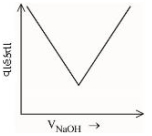
બેન્ઝોઇક એસિડ વિરુદ્ધ, સોડિયમ હાઇડ્રોક્સાઈડના વાહક્તામિતિય અનુમાપનનું સાયું નિરૂપણ પસંદ કરો.
JEE MAIN 2023, Diffcult
b
\(\underset{\text { (wA) }}{ C _6 H _5 COOH }+\underset{\text { (SB) }}{ NaOH } \longrightarrow \underset{\text { (Salt) }}{ C _6 H _5 COONa }+ H _2 O\)
\(\underset{\text { (wA) }}{ C _6 H _5 COOH }+\underset{\text { (SB) }}{ NaOH } \longrightarrow \underset{\text { (Salt) }}{ C _6 H _5 COONa }+ H _2 O\)
\(( A ) \rightarrow( B )\) Free \(H ^{+}\)ions are replaced by \(Na ^{\oplus}\) which decreases conductance.
\((B) \rightarrow (C)\) Un-dissociated benzoic acid reacts with \(NaOH\) and forms salt which increases ions \ conductance increases.
\((C) \rightarrow (D)\) After equivalence point at (3), \(NaOH\) added further increases \(Na ^{\oplus}\) and \(OH ^{\circ}\) ions which further increases the conductance.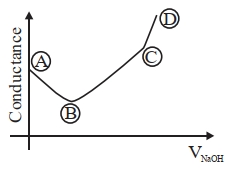
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1હેન્ડેેરસોન સમીકરણ $pH - pK_a= 5$ જે એસિડીક બફર માટે યોગ્ય છે. જ્યારે....View Solution
- 2$K_h$ = $K_w$/$K_a$ નીચેના ...... ક્ષારને લાગુ પડે છે.View Solution
- 3$H^+$ આયન એ ...... છે.View Solution
- 4View Solutionફિનોલ્ફથેલીન.......
- 5$0.1\,M\,CH _{3} COOH$ ના $50\,mL$ નું $0.1\,M\,NaOH$ વિરૂદ્ધ અનુમાપન કરવામાં આવે છે.જ્યારે $25\, mL\, NaOH$ ને ઉમેરવામાં આવે છે ત્યારે દ્રાવણની $pH$ $.......\,\times \,10^{-2}$ થશે. (પૂર્ણાંક જવાબ)View Solution
(આપેલ : $\left.pK _{ a }\left( CH _{3} COOH \right)=4.76\right)$
$\log 2=0.30$
$\log 3=0.48$
$\log 5=0.69$
$\log 7=0.84$
$\log 11=1.04$
- 6View Solutionનીચેનામાંથી કયું એક લુઇસ એસિડ છે ?
- 7${90\,^o}C$ પર શુદ્ધ પાણી $[{H_3}{O^ + }] = {10^{ - 6}}\,M$ છે, તો આ તાપમાન પર ${K_w}$નું મૂલ્ય ...... છે.View Solution
- 8View Solutionનીચેનોમાંથી કયો સૌથી વધુ પ્રબળ સંયુગ્મ બેઇઝ છે?
- 9$25\,°C $ એ પાણીમાં (દળસૂત્ર $= 143) AgCl $ ની દ્રાવ્યતા $ 1.43 \times 10^{-4}$ ગ્રામ$/100 \,ml$ દ્રાવણ હો તો $K_{sp}$ નું મૂલ્ય $= ?$View Solution
- 10$0.1\, M$ દ્રાવણ ઉમેરતા, $Na_2SO_4$ દ્રાવણમાં દરેક $[Ag^+], [Ba^{2+}], [Ca^{2+}]$ના ક્યાં ઘટકો પ્રથમ અવક્ષેપિત છે ?View Solution
$[K_{sp}\, BaSO_4 = 10^{-11}, K_{sp}\, CaSO_4 = 10^{-6}, K_{sp}\,Ag_2SO_4 = 10^{-5}]$