(a)Bomb calorimeter is commonly used to find the heat of combustion of organic substances which consists of a sealed combustion chamber, called a bomb. If a process is run in a sealed container then no expansion or compression is allowed, so \( w = 0 \) and \(\Delta\) \(U = q.\)
\(\Delta\) \(U < 0, w = 0\)
Download our appand get started for free
Similar Questions
- 1${KCl}$ માટે બોર્ન-હેબર ચક્રનું મૂલ્યાંકન નીચેની માહિતી સાથે કરવામાં આવે છે:View Solution
$\Delta_{f} {H}^{\ominus}$ ${KCl}=-436.7 \,{~kJ}\, {~mol}^{-1}$
$\Delta_{\text {sub }} {H}^{\ominus}$ ${K}=89.2 \,{~kJ}\, {~mol}^{-1}$
$\Delta_{\text {ionization }} \,{H}^{-}$ ${K}=419.0\, {~kJ}\, {~mol}^{-1}$
$\Delta_{\text {electron gain }} {H}^{\ominus}$ ${Cl}_{(\text {e) }}=-348.6 \,{~kJ} \,{~mol}^{-1}$
$\Delta_{{bond}} {H}^{-}$ ${Cl}_{2}=243.0 \,{~kJ} \,{~mol}^{-1}$
${KCl}$ની લેટિસ એન્થાલ્પીની તીવ્રતા $.....$ ${kJ} {mol}^{-1}$ છે.
- 2${I_{2\left( s \right)}}$ ની ઊર્ધ્વીકરણ ઊર્જા $57.3\, kJ\, mol^{-1}$ અને ગલન એન્થાલ્પી $15.5\, kJ\,mol^{-1}$ છે. તો ${I_2}$ ની બાષ્પાયન એન્થાલ્પી .....................$kJ\,mo{l^{ - 1}}$ થશે.View Solution
- 3$45.0\, g$ સિલિકોનના તાપમાનમાં $6\,^oC$ નો વધારો કરવા $192\,J$ ઉષ્માની જરૂર પડે તો તેની વિશિષ્ટ ઉષ્માક્ષમતા.....View Solution
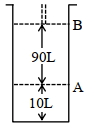
- 4આપેલ આક્રૂતિને ધ્યાનમાં લો.View Solution
$18^{\circ} \mathrm{C}$ પર, સ્થાન $A$ પર, પિસ્ટન સાથે જોડેલા (fitted) સિલિન્ડર માં આદર્શ વાયુનો $1$ $\mathrm{mol}$ રાખેલ છે. જો તાપમાન માં કોઈપણ જાતનો ફેરફાર ન કરીએ તો પિસ્ટન એ સ્થાન $B$ તરફ ખસે છે ત્યારે આ પ્રતિવર્તી પ્રક્રમ માં થયેલ કાર્ય $'x' L atm$ છે. $x=-$ ........... $L.atm$ (નજીક નો પૂર્ણાક)
[આપેલ : નિરપેક્ષ તાપમાન $={ }^{\circ} \mathrm{C}+273.15, \mathrm{R}=0.08206 \mathrm{~L} \mathrm{~atm} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$ ]
- 5એક પ્રણાલીને $60\, J$ ઉષ્મા આપતા તે $15\, J$ કાર્ય કરે છે. તો પ્રણાલીની આંતરિક ઊર્જામાં થતો ફેરફાર.........$J$ થશે.View Solution
- 6$H_2$ $_{(g)}$ + $Br_2$ $_{(g)}$ $\rightarrow$ $2HBr_{(g)}$ પ્રક્રિયા માટે એન્થાલ્પી પરિવર્તનની ગણતરી .....$KJ$ થશે. $H - H, Br - Br$ અને $H - Br$ ની બંધ ઊર્જા અનુક્રમે $435, 192$ અને $364\, kJ \,mol^{-1}$ છે.View Solution
- 7$H_2$$_{(g)} +$ $ \frac{1}{2}O_2$$_{(g)}$ $\rightarrow$ $H_2O$$_{(l)}$ ; $\Delta H = - 68.39 \,KcalK$ $_{(s)} +$ $H_2O$$_{(l)} + aq$ $\rightarrow$ $KOH$ $_{(aq)} + $ $1/2H_2$$_{(g)}$; $\Delta H = - 48.0\, Kcal,$ $ KOH$ $_{(s)} + aq$. $\rightarrow$ $KOH$$_{(aq)}$; $\Delta$$H$ $= - 14.0 \,Kcal$ તો $KOH$$_{(s)}$ ની નિર્માણ ઉષ્મા.....View Solution
- 8નીચેનામાંથી વિશિષ્ટ ગુણધર્મો કયા છે ?View Solution
$(i)$ મોલર વાહકતા $(ii)$ વિધૂત ચાલકબળ $ (iii)$ અવરોધ $(iv)$ ઉષ્માક્ષમતા
- 9નાઈટ્રોજનના પરિમાપન માટે લીધેલા $0.3 $ ગ્રામ કાર્બનિક સંયોજનમાંથી ઉદભવતા એમોનિયાને $100 \,mL$ $ 0.1$ $M\, H_2SO_4$ માંથી પસાર કરવામાં આવે છે. વધારાનું એસિડનું સંપૂર્ણ તટસ્થીકરણ કરવા માટે $ 20\, mL\, 0.5 $ $M\, NaOH$ ની જરૂર પડે છે. આકાર્બનિક પદાર્થ કયો હશે ?View Solution
- 10સમતાપી પરિસ્થિતિમાં $300 \;\mathrm{K}$ પર તેમજ $10^{5}\; \mathrm{Nm}^{-2} $ ના અચળ દબાણના લીધે એક વાયુ $10^{-3} \;\mathrm{m}^{3}$ માંથી $10^{-2} \;\mathrm{m}^{3}$માં વિસ્તરણ પામે છે. તો વાયુ વડે થતુ કાર્ય શું હશે ?View Solution
