$ \Delta \mathrm{H}_{\text {vap }}^0 \mathrm{CCl}_4=30.5 \mathrm{~kJ} / \mathrm{mol} $
$ \text { Mass of } \mathrm{CCl}_4=284 \mathrm{gm} $
$ \text { Molar mass of } \mathrm{CCl}_4=154 \mathrm{~g} / \mathrm{mol} $
$ \text { Moles of } \mathrm{CCl}_4=\frac{284}{154}=1.844 \mathrm{~mol} $
$ \Delta \mathrm{H}_{\text {vap }}{ }^{\circ} \text { for } 1 \mathrm{~mole}=30.5 \mathrm{~kJ} / \mathrm{mol} $
$ \Delta \mathrm{H}_{\text {vap }}{ }^{\circ} \text { for } 1.844 \mathrm{~mol}=30.5 \times 1.844 $
$ \quad=56.242 \mathrm{~kJ}$
Download our appand get started for free
Similar Questions
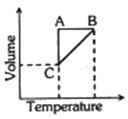
- 1કોઈ વાયુના પાંચ મોલને શ્રેણીમાં થતા ફેરફારના ઘટનાક્રમમાં મૂકવામાં આવેલ છે. જે નીચે આપેલા આલેખ વડે દર્શાવી શકાય છે, તો આ આલેખમાં $A$ $\rightarrow$ $B$, $B$ $\rightarrow$ $C$ અને $C$ $\rightarrow$ $A$ અનુક્રમે શું હશે ?View Solution
- 2એક બોમ્બ કેલોરીમીટરમાં, સાયનામાઈડ $NH _{2} CN _{( s )}$ અને ઓકિસજનની પ્રક્રિયા કરવામાં આવે છે અને તેનો $\Delta U -742.24\, kJ\, mol ^{-1}$ માલૂમ પડયો. તો આપેલી પ્રક્રિયા માટે,View Solution
$NH _{2} CN _{( s )}+\frac{3}{2} O _{2}( g ) \rightarrow N _{2( g )}+ O _{2}( g )+ H _{2} O _{(l)}$
$\Delta H _{298}$ ની માત્રા ........ $kJ$ છે. (નજીક પૂર્ણાંક રાઉન્ડ ઓફ)
[ધારી લો આદર્શ વાયુઓ અને $\left. R =8.314\, J\, mol ^{-1} K ^{-1}\right]$
- 3$H_2$$O$ $_{(l)}$ $(1 \,bar, 373 \,K)$ $\rightarrow$ $H_2O$ $_{(g)}$ $(1 \,bar, 373\, K)$, પ્રક્રિયા માટે ઉષ્માગતિશાસ્ત્રના પરિબળનો સાચો ક્રમ .......View Solution
- 4નીચેનામાંથી કયું સમીકરણ $CH_4$ ની પ્રમાણીત નિર્માણ ઉષ્મા દર્શાવે છે ?View Solution
- 5નીચે આપેલામાંથી માત્રાત્મક ગુણધર્મો ધરાવતા હોય તેની કુલ સંખ્યા $.........$ છે. કદ, મોલર ઉષ્માક્ષમતા, મોલારીટી, $E ^{\varnothing}$ કોષ,ગિબ્ગીસ મુક્ત ઊર્જા ફેરફાર, મોલર દળ, મોલView Solution
- 6$298\, K$ તાપમાને ${H_2}{O_{\left( g \right)}}$ ની પ્રમાણિત સર્જન એન્થાલ્પી $- 241.82\, kJ\, mol^{-1}$ છે. જો $C_p$ તાપમાનથી સ્વતંત્ર હોય તો $373\, K$ તાપમાને ${H_2}{O_{\left( g \right)}}$ ની સર્જન એન્થાલ્પી જણાવો.View Solution
${C_p}$ of ${H_2}{O_{\left( g \right)}}$ $ = 33.58\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$
${C_p}$ of ${H_{2\left( g \right)}}$ $ = 28.84\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$
${C_p}$ of ${O_{2\left( g \right)}}$ $ = 29.37\,J\,{K^{ - 1}}\,mo{l^{ - 1}}$
- 7$X_2, Y_2$ અને $XY_3$ ના પ્રમાણિત એન્ટ્રોપી મૂલ્યો અનુક્રમે $60, 40$ અને $50\, J \,K^{-1}\, mol^{-1}$ છે. પ્રક્રિયા $\frac{1}{2} \, X_2 + \frac{3}{2} \,Y_2 \rightleftharpoons XY_3,$ માટે $ \Delta H = -30 \,kJ$ સંતુલન સ્થિતિ માટે ............... $\mathrm{K}$ તાપમાન જરૂરી છે ?View Solution
- 8એસિડ-બેઈઝ પ્રક્રિયાની તટસ્થીકરણ ઉષ્મા $56$ કિલોજૂલ પ્રતિ મોલ હોય, તો તે પદાર્થો ...... હોઈ શકે.View Solution
- 9View Solutionસમોષ્મી પ્રક્રિયા માટે નીચેનામાંથી કયો સંબંધ સાચો છે ?
- 10એક મોલ આદર્શવાયુ $1$ વાતાવરણદબાણે $10$ લીટર ક્ષમતા ધરાવતા બલ્બમાં ભરવામાં આવે અને $100$ લીટર ક્ષમતાનો નિર્વાતન બલ્બમાં દાખલ કરવામાં આવે છે. આ કિસ્સામાં કેટલા .... જુલ કાર્ય પૂર્ણ થયું ?View Solution
