$C{H_4} + C{l_2}\xrightarrow{{hv}}C{H_3}Cl + HCl$
$ CH_3Cl $ ની ઉપજ મેળવવા માટે, $ CH_4 $ થી $ Cl_2 $નો ગુણોત્તર કેવો હોવો આવશ્યક છે ?
Medium
a
\((a)\) In order to maximize the amount of monohalogenated product obtained,a radical substitution reaction should be carried out in the presence of excess of alkane. The presence of excess alkane in the reaction mixture ensures that there is a greater probability of the halogen radical colliding with a molecule of alkane than with a molecule of alkyl halide. This is true even toward . the end of the reaction, by which time a considerable amount of alkyl halide will have been formed. If the halogen radical abstracts a hydrogen from a molecule of alkyl halide rather than from a molecule of alkane, a dihalogenated product will be obtained
\((a)\) In order to maximize the amount of monohalogenated product obtained,a radical substitution reaction should be carried out in the presence of excess of alkane. The presence of excess alkane in the reaction mixture ensures that there is a greater probability of the halogen radical colliding with a molecule of alkane than with a molecule of alkyl halide. This is true even toward . the end of the reaction, by which time a considerable amount of alkyl halide will have been formed. If the halogen radical abstracts a hydrogen from a molecule of alkyl halide rather than from a molecule of alkane, a dihalogenated product will be obtained
\(C{l^ \bullet } + C{H_3}Cl \to {\,^ \bullet }C{H_2}Cl + HCl\)
\(^ \bullet C{H_2}Cl + C^{12} \to C{H_2}C{l_2} + C{l^ \bullet }\)
Bromination of alkanes follows the same mechanism as chlorination. The only difference is that chlorination produces alkyl chlorides, whereas bromination forms alkyl bromides
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચે બતાવેલ $3,3$ - ડાયમિથાઇલ - $1$ -બ્યુટીન ના વધારાને ધ્યાનમાં લો.નિરીક્ષણ કરેલ નીપજ ની રચના માટે શ્રેષ્ઠ પદ્ધતિઓની સમજૂતી શું છે?View Solution
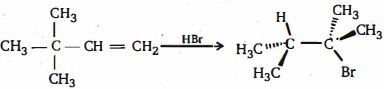
- 2View Solutionનીપજ ની અવકાશીયરસાયણ શું છે ?
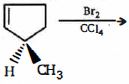
- 3View Solutionનીચેની પ્રકિયામાંથી સાચી નીપજ કઈ છે ?
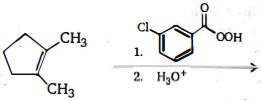
- 4$C_6H_{10}$ હાઈડ્રોકાર્બન ઉદ્દીપક હાઈડ્રોજીનેશનમાં $H_2$ નો માત્ર એક અણુનું શોષણ કરે છે. જેના પર ઓઝોનોલીસીસ કરતા, હાઈડ્રોકાર્બનની ઉપજ $CHO(CH_2)_4CHO$ નીપજ આવે છે. હાઈડ્રોકાર્બન કયો છે ?View Solution
- 5પદાર્થ $'A'$ પર ક્લોરીનેશનથી પદાર્થ $'B'$ આપે છે. પદાર્થ $'B'$ ને આલ્કોહોલીક $KOH$ સાથે પ્રક્રિયા કરતા પદાર્થ $'C'$ આપે છે. કે જે બેયરના પ્રક્રીયકને રંગવિહીન કરે છે. પદાર્થ $ 'C'$ નું ઓઝૉનોલીસીસથી $HCHO$ આપે છે પદાર્થ $ 'A'$ શું હશે ?View Solution
- 6$C_9H_{18}$ અણુસૂત્ર ધરાવતા આલ્કીનના ઓઝોનોલિસિસથી $2, 2-$ ડાયમિથાઇલ પ્રોપેનાલ અને $2-$ બ્યુટેનોન મળે છે. તો આલ્કીન .................... છે.View Solution
- 7View Solutionનીચેનામાંથી કોણ એસિટિલિન સાથે પ્રક્રિયા કરશે નહિ ?
- 8એક આલ્કાઇલ બ્રોમાઇડ $(X)$ ની $Na$ સાથેની પ્રક્રિયાથી $4, 5-$ ડાયઇથાઇલ ઓક્ટેન મળે છે. તો $X$............. થશે.View Solution
- 9નીચે બે વિધાનો આપેલા છે. એકને કથન $A$ અને બીજાને કારણ $R$ વડે લેબલ કરેલ છે.View Solution
કથન $A$ : $[6]$ એન્યુલીન, $[8]$ એન્યુલીન, સિસ-$[10]$ એન્યુલીન અને ટ્રાન્સ -$[10]$ એન્યુલીન ક્રમશઃ એરોમેટિક, નોન-. એરોમેટિક, એરોમેટિક અને નોન-એરોમેટિક છે
કારણ $R$ : એરોમેટિક અને એન્ટી એરોમેટિક પ્રણાલી માટે સમતલીયતા એ એક જરૂરિયાત છે.
ઉપરોક્ત વિધાનોના સંદર્ભે આપેલા વિકલ્પોમાંથી યોગ્ય ઉત્તર પસંદ કરો
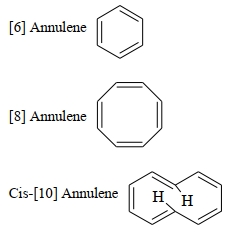
- 10બેન્ઝિનમાં $C - C$ બંધની લંબાઇ ......$\mathop A\limits^o $ છે.View Solution
