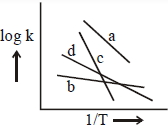
\(\log \mathrm{K}=\frac{-\mathrm{Ea}}{2.303 \mathrm{RT}}+\log \mathrm{A}\)
Acrroding to Arrhenius equation plot of \(\operatorname{log } \mathrm { K }\) Vs. \(\frac{1}{\mathrm{T}}\) is linear with.
slope \(=\frac{-\mathrm{Ea}}{2.303 \mathrm{R}}\)
From plot we conclude:
\(|\) slope \(| \quad: c>a>d>b\)
\(\therefore \mathrm{E}_{\mathrm{c}}>\mathrm{E}_{\mathrm{a}}>\mathrm{E}_{\mathrm{d}}>\mathrm{E}_{\mathrm{b}}\)
Download our appand get started for free
Similar Questions
- 1$aG + bH \rightarrow$ નિપજ પ્રક્રિયાને ધ્યાનમાં લેતાં જ્યારે $G$ અને $H$ બંને પ્રક્રિયકોની સાંદ્રતા બમણી હોય તો દર વધીને $8$ ગણું થાય છે. જો કે જ્યારે $G$ ની સાંદ્રતા બમણી થાય ત્યારે $H$ ની સાંદ્રતા નિયત રહે તો દર બમણો થશે. તો સમગ્ર પ્રક્રિયાનો ક્રમ શું થશે?View Solution
- 2જો $87.5\% $ નો કોઈપણ આપેલ પદાર્થનું $40$ મિનિટમાં વિભંજન થાય તો રેડીયો એક્ટિવ પદાર્થની અદ્ય આયુ કેટલી થાય છે?View Solution
- 3પ્રક્રિયા પ્રણાલી $2NO(g) + {O_2}(g) \to 2N{O_2}(g)$ માટે દબાણ વધારીને એકાએક તેનું કદ અડધુ કરવામાં આવે છે. જો પ્રક્રિયા $O_2$ ના સંદર્ભમાં પ્રથમ ક્રમની અને $NO$ ના સંદર્ભમાં દ્વિતીય ક્રમની હોય, તો પ્રક્રિયાનો વેગ ....View Solution
- 4નીચે આપેલ બે જુદી જુદી પ્રથમ ક્રમ પ્રક્રિયા ને ધ્યાનમાં લોView Solution
$\mathrm{A}+\mathrm{B} \rightarrow \mathrm{C}$ (પ્રક્રિયા $1)$
$\mathrm{P} \rightarrow \mathrm{Q}$ (પ્રક્રિયા $2$)
પ્રક્રિયા $1$ : પ્રક્રિયા $2$ ના અર્ધં આયુષ્ય નો ગુણોત્તર $5: 2$ છે. પ્રક્રિયા $1$ અને પ્રક્રિયા $2$ ને $2 / 3^{\text {dd }}$ and $4 / 5^{\text {dd }}$ પૂર્ણ થવા માટે લાગતા સમયને અનુક્રમે $t_1$ અને $t_2$ તરીકે રજૂ કરવા આવે તો $t_1: t_2$ ગુણોત્તર નું મૂલ્ય ........... $\times 10^{-1}$ છે. (નજીક નો પૂર્ણાક)
[આપેલ : $\log _{10}(3)=0.477$ અને $\log _{10}(5)=0.699$ ]
- 5જ્યારે તાપમાન વધીને $300\,K$ થી $310 \,K$ થાય ત્યારે પ્રક્રિયાનો દર $2.3 $ ગણુ વધે છે. જો $300 \,K$ એ દર અચળાંક $x$ હોય તો $310 \,K$ એ દર અચળાંક....... જેટલું થાય છે.View Solution
- 6એક પ્રક્રિયાની સક્રિયકરણ શક્તિ જે $80.9 \,kJ \,mol^{-1}$ છે. તેમાં અણુઓનો એક અંશ (ભાગ) જે $700\, K$ પર, પ્રક્રિયા કરીને નીપજ બનવા માટે પૂરતા પ્રમાણમાં ઊર્જા ધરાવે છે તે $e ^{-x}$ છે. તો $x$નું મૂલ્ય ..... છે.View Solution
(નજીકના પૂર્ણાંકમાં રાઉન્ડ ઑફ) $[$ ઉપયોગ કરો : $\left. R =8.31 \,J \,K ^{-1} \,mol ^{-1}\right]$
- 7જો પ્રક્રિયક $ B$ ની સાંદ્રતા બમણી થાય તો પ્રક્રિયક $A$ અને $B$ વચ્ચેની પ્રક્રિયાનો દર પ્રારંભિક દર $1/4$ જેટલો થાય છે. પ્રક્રિયક $B$ ના સંદર્ભમાં પ્રક્રિયાનો ક્રમ ...... થશે.View Solution
- 8પ્રક્રિયા${H_{2\left( g \right)}} + {I_{2\left( g \right)}} \to 2H{I_{\left( g \right)}}$ માટેની શક્ય ક્રિયાવિધિ નીચે મુજબ છે.View Solution
${I_2}\,\underset{{{K_{ - 1}}}}{\overset{{{K_1}}}{\longleftrightarrow}}\,2I\,$ (fast step)
$2I + {H_2}\xrightarrow{{{K_2}}}2HI$ (slow step)
તો પ્રક્રિયાનો વેગનિયમ જણાવો.
- 9એક પ્રક્રિયા $2$ કલાકમાં $50 \%$ પૂર્ણ થાય છે. તથા $4$ કલાક માં $75 \%$ પૂર્ણ થાય છે. તો પ્રક્રિયાનો ક્રમ ..........View Solution
- 10View Solutionનીચેનામાંથી ક્યુ વિધાન ખોટુ છે ?
