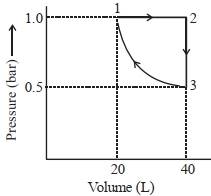
\(1 \rightarrow 2 \Rightarrow \text { Isobaric process }\)
\(2 \rightarrow 3 \Rightarrow \text { Isochoric process }\)
\(3 \rightarrow 1 \Rightarrow \text { Isothermal process }\)
\(W = W _{1 \rightarrow 2}+ W _{2 \rightarrow 3}+ W _{3 \rightarrow 1}\)
\(=\left(- P \left( V _2- V _1\right)+0\left[- P _1 V _1 \ln \left(\frac{ V _2}{ V _1}\right)\right]\right)\)
\(=\left[-1 \times(40-20)+0+\left[-1 \times 20 \ln \left(\frac{20}{40}\right)\right]\right]\)
\(=-20+20 \ln 2\)
\(=-20+20 \times 2.3 \times 0.3\)
\(=-6.2 \text { bar } L\)
\(| W |=6.2 \text { bar } 1=620\,J\)
Download our appand get started for free
Similar Questions
- 1View Solutionકોની ઉર્જા નક્કી કરવા માટે હેસનો નિયમ લાગુ પડે છે
- 2જ્યારે $2$ મોલ $C_2H_6$ સંપૂર્ણ પણે સળગી $3129\, kJ$ ઉષ્મા છૂટી પાડે છે. તો $C_2H_6$ ની નિર્માણ ઉષ્મા .....$J$ થશે. $CO_2$ અને $H_2O$ ની $\Delta \,Hf$ અનુક્રમે $-395$ અને $-286 \,kJ$ છે.View Solution
- 3આપેલ ,View Solution
$(i)\,\,C\,({\rm{graphite}})\, + \,{O_2}{\kern 1pt} (g)\, \to \,C{O_2}\,(g);\,\Delta r{H^\circleddash} = x\,\,kJ\,mo{l^{ - 1}}$
$(ii)\,\,C\,({\rm{graphite}})\, + \,\frac{1}{2}{O_2}{\kern 1pt} (g)\, \to \,CO\,(g);\,\Delta r{H^\circleddash} = y\,\,kJ\,mo{l^{ - 1}}$
$(iii)\,\,CO\,(g)\, + \,\frac{1}{2}{O_2}{\kern 1pt} (g)\, \to \,C{O_2}\,(g);\,\Delta r{H^\circleddash} = z\,\,kJ\,mo{l^{ - 1}}$
ઉપરોક્ત, ઊષ્મારાસાયણિક સમીકરણો ના આધારે નીચેનામાંથી ક્યો બીજગણિતિક સંબંધ સાચો છે?
- 4${I_{2\left( s \right)}}$ ની ઊર્ધ્વીકરણ ઊર્જા $57.3\, kJ\, mol^{-1}$ અને ગલન એન્થાલ્પી $15.5\, kJ\,mol^{-1}$ છે. તો ${I_2}$ ની બાષ્પાયન એન્થાલ્પી .....................$kJ\,mo{l^{ - 1}}$ થશે.View Solution
- 5View Solutionકોની ઉર્જા નક્કી કરવા માટે હેસનો નિયમ લાગુ પડે છે
- 6જ્યારે અચળ દબાણમાં $4 \,g$ આયર્નને સળગાવતા ફેરિક ઓક્સાઈડમાં રૂપાંતર થાય છે,ત્યારે $29.28\, kJ $ ઉષ્માનું નિર્માણ થાય છે. ફેરિક ઓક્સાઇડની સર્જન એન્થાલ્પીનું મૂલ્ય ......$kJ$ છે.(પરમાણ્વીય ભાર $Fe = 56$ )View Solution
- 7View Solutionદરેક તાપમાને સ્વયંભૂ પ્રક્રિયા માટે સાચી ઉષ્માગતિકીય પરિસ્થિતિઓ ........
- 8View Solutionસ્વયંભૂ થવાની પ્રક્રિયા માટે
- 9નીચેનામાંથી કયા સમીકરણો મિથેનની સર્જન પ્રમાણિત ઉષ્મા $(\Delta H_f^o)$ ને યોગ્ય રીતે રજૂ કરે છે?View Solution
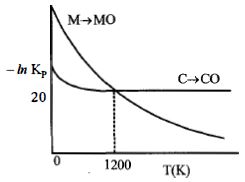
- 10નીચેનો આલેખ એ પ્રક્રિયાઓ $M(s) + \frac{1}{2}{O_2}(s)\, \to \,MO(s)\,$ અને $C(s) + \frac{1}{2}{O_2}(g)\, \to \,CO(s)\,$ માટે $- In\,K_p$ વિરુદ્ધ તાપમાનનો ફેરફાર દર્શાવે છે. તો સાયુ વિધાને ઓળખો.View Solution