એસિડિક પ્રબળતામાં વધારો કરવાના ક્રમ આપો

Medium
c
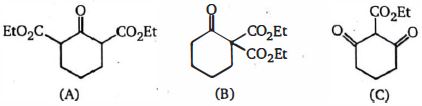
The greater $I$ effect is favorable or greater acidic strength. The $-I$ effect of aldehyde group is greater than the ester group. Due to this $C$ will be most acidic. In $A$ and $B , B$ will be less acidic as it has $-I$ effect sort of localized due to ester group being on a single carbon. In $A$ it is more delocalized is not effective on more by adjacent carbons.
The greater $I$ effect is favorable or greater acidic strength. The $-I$ effect of aldehyde group is greater than the ester group. Due to this $C$ will be most acidic. In $A$ and $B , B$ will be less acidic as it has $-I$ effect sort of localized due to ester group being on a single carbon. In $A$ it is more delocalized is not effective on more by adjacent carbons.
Hence, the required order is- $B\, <\, A\, <\, C$.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1નીચે આપેલામાંથી કોના કારણે તૃતીયક બ્યુટાઇલ કાર્બોકેટાયન એ દ્વિતીયક બ્યુટાઇલ કાર્બોકેટાયન કરતા વધુ સ્થાયી છે $?$View Solution
- 2View Solutionનીચે આપેલા પૈકી ક્યો કાર્બોનેટાયન સૌથી વધુ સ્થાયી હોવાની અપેક્ષા રાખી શકાય ?
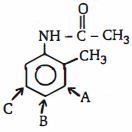
- 3ઇલેક્ટ્રોનઅનુરાગી ચક્રીયવિસ્થાપન $(EAS)$ સૌથી વધુ અનુકૂળ છે તે સ્થાનને ઓળખો.View Solution
- 4View Solutionનીચેનામાંથી કયો પ્રબળ બેઈઝ છે ?
- 5નીચે આપેલા નિશાનીવાળા પ્રોટોનમાંથી ક્યું સંયોજન $pK _{ a }$ મૂલ્ય સૌથી ઓછુ પ્રદર્શિત કરે છે ?View Solution
- 6$EAS$ (ઇલેક્ટ્રોન અનુરાગી વિસ્થાપન ) તરફ ઘટાડા નો સાચો ક્રમ કયો છેView Solution
- 7બેઇઝ પ્રબળતાના ઘટતા ક્રમમાં નીચેના ક્રમ આપોView Solution
$(A)\, CH_3 - CH_2 - C \equiv C^-$ $(B) \,CH_3 -CH_2 - S^-$
$(C) \,CH_3 - CH_2 - CO^-_2$ $(D)\, CH_3 -CH_2 - O^-$ - 8View Solutionઆપેલ પ્રક્રિયાને કઇ ઉર્જા આકૃતિ શ્રેષ્ઠ રજૂ કરે છે?
- 9એનીલીન $(I)$, બેન્ઝીન $(II)$ નાઈટ્રોબેન્ઝિન $(III)$ પદાર્થોના ઈલેકટ્રોન અનુરાગી વિસ્થાપનનો સાચો ક્રમ કયો છે ?View Solution
- 10નીચેનામાંથી કયો સેન્ડ મેયર પ્રકિયા માં મધ્યવર્તી શું હશે ?View Solution
$(i)$ ${C_6}{H_5}{N^ + } \equiv NC{l^ - }$ $(ii)$ ${C_6}{H_5}{N^ + } \equiv N$
$(iii)$ ${{\overset{\centerdot }{\mathop{C}}\,}_{6}}{{H}_{5}}$ $(iv)$ $C_6H_5Cl$
