આપેલ ધનાયનમાં, સૌથી સ્થાયી કાર્બોનિયમ આયન કયો છે?
IIT 1981, Medium
b
(b) \(\underset{\,\,\,\,\,\,\,\,\,{{3}^{o}}}{\mathop{C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{\,\,C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,}{\mathop{{{C}^{+}}\,}}}\,}}}\,>}}\,\,\underset{\,\,\,\,\,\,\,\,\,\,\,2{}^\circ }{\mathop{C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH{{_{{}}^{{}}}^{+}}}}\,}}\,>}}\,\,\underset{\begin{matrix}
\begin{matrix}
{} \\
\begin{matrix}
{} \\
{{1}^{o}} \\
\end{matrix} \\
\end{matrix} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
\end{matrix}}{\mathop{C{{H}_{3}}-CH{{_{2}^{{}}}^{+}}}}\,>\underset{\begin{matrix}
{} \\
\begin{matrix}
{} \\
\begin{smallmatrix}
\text{Methyl } \\
\text{carbocation}
\end{smallmatrix} \\
\end{matrix} \\
\end{matrix}}{\mathop{CH{{_{3}^{{}}}^{+}}\,\,\,\,\,\,\,}}\,\)
(b) \(\underset{\,\,\,\,\,\,\,\,\,{{3}^{o}}}{\mathop{C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{\,\,C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,}{\mathop{{{C}^{+}}\,}}}\,}}}\,>}}\,\,\underset{\,\,\,\,\,\,\,\,\,\,\,2{}^\circ }{\mathop{C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH{{_{{}}^{{}}}^{+}}}}\,}}\,>}}\,\,\underset{\begin{matrix}
\begin{matrix}
{} \\
\begin{matrix}
{} \\
{{1}^{o}} \\
\end{matrix} \\
\end{matrix} \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
\end{matrix}}{\mathop{C{{H}_{3}}-CH{{_{2}^{{}}}^{+}}}}\,>\underset{\begin{matrix}
{} \\
\begin{matrix}
{} \\
\begin{smallmatrix}
\text{Methyl } \\
\text{carbocation}
\end{smallmatrix} \\
\end{matrix} \\
\end{matrix}}{\mathop{CH{{_{3}^{{}}}^{+}}\,\,\,\,\,\,\,}}\,\)
The higher the number of alkaline groups, the greater the charge is the deflection and hence the more carbonium ion is permanent.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionકાર્બએનાયન ની સ્થિરતા નો સાચો ક્રમ શોધો.
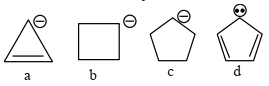
- 2View Solutionકયો સૌથી એસિડિક પદાર્થ છે ?
- 3નીચે બે વિધાનો આપવામાં આવ્યા છે:View Solution
વિધાન $I : C _{2} H _{5} OH$ અને $AgCN$ બંને કેન્દ્રાનુરાગી ઉત્પન્ન કરી શકે છે.
વિધાન $II : KCN$ અને $AgCN$ બંને બધી પ્રક્રિયાની પરિસ્થિતિઓ સાથે નાઇટ્રિલ કેન્દ્રાનુરાગી ઉત્પન્ન કરશે.
સૌથી યોગ્ય વિકલ્પ પસંદ કરો:
- 4$2-$બ્રોમોબ્યુટેન માંથી બ્રોમિનની વિલોપન રચનામાં પરિણમે છેView Solution
- 5View Solutionએસિડ પ્રબળતા ઓછી થવાના ક્રમમાં નીચેના સંયોજનો ક્રમ આપો મોટાભાગે એસિડિક થી ઓછામાં ઓછું એસિડિક)
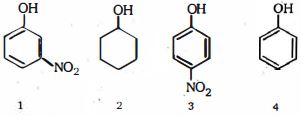
- 6નીચેના કયા સંયોજન ની $pK_a$ ની મૂલ્ય ઊંચી છે ?View Solution
- 7નીચેની બેન્ઝાઈલ/એલાઈલ પ્રણાલીમાં ( $R - $આલ્કાઈલ સમૂહ) પ્રેરક અસરનો ઘટતો ક્રમ કયો છે ?View Solution
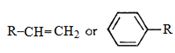
- 8નીચેના $(I-III)$ સંયોજનોમાં ઇલેક્ટ્રોન અનુરાગી પ્રક્રિયક સાથે પ્રક્રિયાનો યોગ્ય ક્રમ છે?View Solution
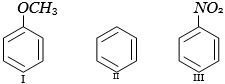
- 9પીક્રીક એસિડ, એસિટિક એસિડ અને ફિનોલના $ H_2O$ માં $ pK_a $ મૂલ્ય કયા ક્રમમાં છેView Solution
- 10View Solutionનીચેનામાંથી કયું ઇલેક્ટ્રોન અનુરાગી પ્રક્રિયક પ્રત્યે સૌથી વધુ પ્રતિક્રિયાશીલ છે?
