$\,\,\frac{1}{2}\,\,{H_2} + \,\,\frac{1}{2}\,\,C{l_2}\, \to \,\,HCl$
$\Delta H\,\, = \,\,\,\frac{1}{2}\,\, \times \,\,434\,\, + \,\,\frac{1}{2}\, \times \,\,242 - 431\,\, = \,\,217\,\, + \,\,121\,\, - \,\,431\,\, = \,\, - 93\,\,KJ/mol$
Download our appand get started for free
Similar Questions
- 1નીચેની માહિતી પરથી સુક્રોઝના $ (C_{12}H_{22}O_{11})$ નિર્માણ એન્થાલ્પીની ગણતરી ............. $\mathrm{JK}^{-1} \, \mathrm{mol}^{-1}$ થશે.View Solution
$(i)\,\,{C_{12}}{H_{22}}{O_{11}}\,\, + \,\,12{O_2}\,\, \to \,\,12\,\,C{O_2}\, + \,\,11{H_2}O,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H\,\, = \,\, - 5200.7\,kJ\,mo{l^{ - 1}} $
$(ii)\,\,C\,\, + \,\,{O_2}\, \to \,\,C{O_2},\,\,\,\,\,\,\,\,\,\,\,\,\Delta H\,\, = \,\, - \,394.5\,\,kJ\,\,mo{l^{ - 1}}$
$(iii)\,\,{H_2}\,\, + \,\frac{1}{2}{O_2}\,\, \to \,\,\,{H_2}O,\,\,\,\,\,\,\,\,\,\Delta H\,\, = \,\, - \,285.8\,kJ\,\,mo{l^{ - 1}}$
- 2$250\,^oC$ એ સુક્રોઝ ($C_{12}H_{22}O_{11}$) ના દહન માટે કયું સાચું છે ?View Solution
- 3નીચેની બે પ્રક્રિયાઓ જાણીતી છે.View Solution
$Fe_2O_{3(s)} + 3CO_{(g)} \rightarrow 2Fe_{(s)} + 3CO_{2(g)};$ $\Delta H = - 26.8\, kJ$
$FeO_{(s)} + CO_{(g)} \rightarrow Fe_{(s)} + CO_{2(g)} \, ;$ $\Delta H = - 16.5\, kJ$
નીચેની પ્રક્રિયા માટે $\Delta H$ નું મૂલ્ય કેટલા ............. $\mathrm{kJ}$ થશે ?
$Fe_2O_{3(s)} + CO_{(g)} \rightarrow 2FeO_{(s)} + CO_{2(g)}$ is
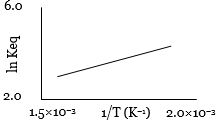
- 4એક પ્રક્રિયા માટે $\ln K_{eq}$ વિરુદ્ધ તાપમાનના વ્યસ્તનો આલેખ નીચે મુજબ છે. તો પ્રક્રિયા ........ હશે.View Solution
- 5પાણી માટે $\Delta {H_{vap}}$ મૂલ્ય $40.73\, kJ\, mol^{-1}$ અને $\Delta {S_{vap}}$ નુ મૂલ્ય $109\, J\,K^{-1}\,mol^{-1}$ છે. તો ક્યા તાપમાને પાણી તેની બાષ્પ સાથે સંતુલનમાં હશે ?View Solution
- 6અચળ તાપમાને અને દબાણે નીચેનામાંથી કયું વિધાન સમીકરણ માટે સાચું છે ?View Solution
$CO_{(g)} + \frac{1}{2} \,O_{2(g)}\rightarrow CO_{2(g)}$ અચળ તાપમાન અને દબાણ
- 7View Solutionઉષ્માગતિકીય ફેરફારોમાં નીચેના પૈકી ક્યુ વિધાન/સંબંધ સાચું નથી ?
- 8$H_{2(g)} + I_{2{(g)}} \rightarrow 2HI; \Delta H = 12.40\, Kcal$. પ્રક્રિયાના આધારે $HI$ ની નિર્માણ ઉષ્મા.......$Kcal$View Solution
- 9નીચે આપેલા પૈકી કર્યું એક આદર્શ વાયુના એક મોલ માટે $\mathrm{C}_{\mathrm{P}}$ અને $\mathrm{C}_{\mathrm{V}}$ વચ્ચે સાચા સંબંધ માટેનો સાચો વિકલ્પ દર્શાવે છે ?View Solution
- 10સુક્રોઝ $(C_{12}H_{22}O_{11})$ ની દહનઉષ્મા $1350$ કિલો કેલેરી મોલ$^{-1}$ $17.1 $ ગ્રામ સુક્રોઝના દહનથી કેટલા .....કિલો કેલેરી ઉષ્મા મુક્ત થશે ?View Solution
