${H_2}S$ અણુ માં ક્યાં પ્રકાર નો બંધ હાજર છે
Medium
b
(b)Electronic configuration of \(_{16}{S^{32}} = 1{s^2},2{s^2},2{p^6},3{s^2},3{p^4}\). In the last orbit it has only \(6\) electron. So it require \(2\) electron to complete its octet, therefore it share \(2\) electron with two hydrogen atom and forms \(2\) covalent bond with it.
(b)Electronic configuration of \(_{16}{S^{32}} = 1{s^2},2{s^2},2{p^6},3{s^2},3{p^4}\). In the last orbit it has only \(6\) electron. So it require \(2\) electron to complete its octet, therefore it share \(2\) electron with two hydrogen atom and forms \(2\) covalent bond with it.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionસહસંયોજક બંધમાં ..............
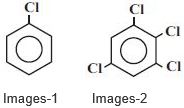
- 2$Images-1$ ની દ્વિધુવ ચાકમાત્રા $1.5 \,D$ હોય, તો $Images-2$ ની દ્વિધુવ ચાકમાત્રા .................. $\mathrm{D}$ થશે.View Solution
- 3$\mathrm{BF}_3, \mathrm{PF}_3$ અને $\mathrm{ClF}_3$ પૈકી બંધખૂણાઓ માટે સાયો ચઢતો ક્રમ શોધો .View Solution
- 4View Solutionનીચેનામાંથી અષ્ટફલકીય ક્યો પદાર્થ/આયન હશે?
- 5View Solutionબે કાર્બન પરમાણુઓ વચ્ચે બનતા સિગ્મા અને પાઇ બંધો માટે નીચેનામાંથી ક્યું વિધાન સાચું નથી?
- 6View Solutionનીચેનામાંથી કોણ સમબંધારણીય જોડ દર્શાવે છે ?
- 7View Solutionનીચે આપેલામાંથી એવું સંયોજન પસંદ કરો કે જે આંત:આણ્વીય હાઈડ્રોજન બંધન દર્શાવે છે.
- 8$CHCl_3, CH_4$ અને $SF_4$ માંથી, કયા અણુઓ નિયમિત ભૂમિતિ ધરાવતા નથી?View Solution
- 9$HF$ માં બંધઅંતર $9.17\times10^{-11}\, m$ છે. $HF$ ની દ્વિધ્રુવ ચાકમાત્રા $6.104\times10^{- 30}\, Cm$ છે, તો $HF$ માં ટકાવાર આયનીય લાક્ષણિકતા જણાવો. (ઇલેક્ટ્રોન વીજભાર $= 1.60\times10^{-19}\, C$)View Solution
- 10View Solution............ દ્વારા ઘન લેટાઈસમાં આયોડિન પરમાણુઓ રાખવામાં આવે છે.
