ઇથેનના સાંતરિત તથા ગ્રસ્ત સંરૂપણ ની સરખામણીના સંદર્ભમાં સાચુ વિધાન .... છે.
NEET 2016, Medium
b
Magnitude of torsional strain depends upon the angle of rotation about $\mathrm{C}-\mathrm{C}$ bond. Staggered form has the least torsional strain and the eclipsed form has the maximum torsional strain. So, the staggered conformation of ethane is more stable than the eclipsed conformation.
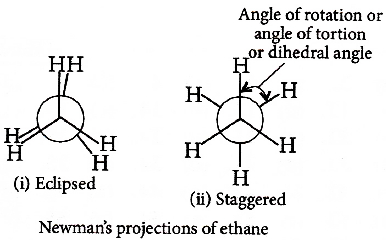
Magnitude of torsional strain depends upon the angle of rotation about $\mathrm{C}-\mathrm{C}$ bond. Staggered form has the least torsional strain and the eclipsed form has the maximum torsional strain. So, the staggered conformation of ethane is more stable than the eclipsed conformation.

Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solution‘અસંમિત ઇથીલિનિક દ્વિબંધ ધરાવતા બે કાર્બન પૈકી ઓછા હાઇડ્રોજન ધરાવતા કાર્બન સાથે પ્રક્રિયકનો વધુ વિદ્યુતઋણીય ઘટક જોડાય છે.’ આ નિયમ..... તરીકે ઓળખાય છે.
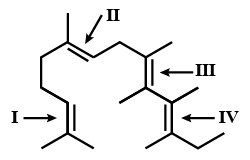
- 2નીચેની કોની રચનામાં, $I$, $II$, $III$ અને $IV$ દ્વિબંધ ચિહ્નિત થયેલ છે?View Solution
- 3$MMPP \to$ મેગ્નેશિયમ મોનો પેરોકસી પ્થેલેટ, નીપજ $(X)$ શું હશે ?View Solution

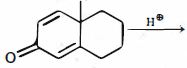
- 4View Solutionમુખ્ય નીપજ........
- 5$HCl$ નો ઉમેરો એ $3,3$ -ડાયમિથાઇલ - $1$ -બ્યુટીન બે ઉત્પાદનો આપે છેજેમાંથી એકમાં ફરીથી ગોઠવાયેલા કાર્બન સ્કેલટન છે. નીચેના કાર્બોકેકેટાયન માં, તે પ્રક્રિયામાં સંભવિત મધ્યસ્થીઓને પસંદ કરોView Solution
$\mathop {{{(C{H_3})}_3}C\mathop C\limits^ + HC{H_2}Cl}\limits_1 $ $\mathop {{{(C{H_3})}_3}C\mathop C\limits^ + HC{H_3}}\limits_2 $ $\mathop {\begin{array}{*{20}{c}}
{{{(C{H_3})}_2}C\mathop C\limits^ + {{(C{H_3})}_2}} \\
{|\,\,\,\,} \\
{Cl\,\,\,}
\end{array}}\limits_3 $ $\mathop {{{(C{H_3})}_2}\mathop C\limits^ + CH{{(C{H_3})}_2}}\limits_4 $ - 6View Solutionએરોમેટિક સંયોજનોના નાઇટ્રેશન અને નીચેના પૈકી ક્યુ વિધાન ખોટું છે ?
- 7નીચે આપેલી રાસાયણિક પ્રક્રિયા ને ધ્યાનમાં લો,View Solution
$CH \equiv CH\xrightarrow[{{\text{(2) CO , HCl , AlC}}{{\text{l}}_3}}]{{{\text{(1) Red hot Fe tube, 873 K}}}}$ નીપજ
નીપજમાં $s p^{2}$ સંકરણ પામેલા કાર્બન પરમાણુઓની સંખ્યા ......... છે.
- 8View Solutionનીચેની પ્રક્રિયાની મુખ્ય નીપજ શું છે?
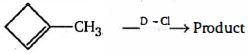
- 9$\begin{array}{*{20}{c}}View Solution
{CH} \\
{|||\,\,\,\,\,} \\
{CH}
\end{array}$ $\xrightarrow{{{O_3}/NaOH}}X\xrightarrow{{Zn/C{H_3}COOH}}Y$ ‘$Y$’ શું છે? - 10નીચેની પ્રક્રિયામાં $X$ શુ હશે ?View Solution
${C_2}{H_2}\xrightarrow[{HgS{O_4}/{H_2}S{O_4},60{\,^o}C}]{{{H_2}O}}X \rightleftharpoons C{H_3}CHO$
