જ્યારે $HOCl$ની સાથે $3-$મિથાઇલ- $2-$પેન્ટીનની પ્રક્રિયા થાય, ત્યારે મુખ્ય નીપજ કઈ રચાય છે?
IIT 1995, Medium
c
(c) \(C{H_3} - C{H_2} - \mathop {\mathop {C{\mkern 1mu} {\mkern 1mu} = {\mkern 1mu} {\mkern 1mu} }\limits_{|{\kern 1pt} \,\,\,\,\,{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} } }\limits_{C{H_{3\,\,\,}}} \mathop {CH - C{H_3} + \mathop {HOCl}\limits^{{\kern 1pt} - {\kern 1pt} {\kern 1pt} {\kern 1pt} + } }\limits_{\mathop {{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} }\limits_{} } \to C{H_3} - C{H_2} - \mathop {\mathop {C{\mkern 1mu} {\mkern 1mu} - }\limits_{|{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} }^{|{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} } }\limits_{{\kern 1pt} C{H_3}}^{OH} \mathop {CH}\limits^{\mathop {|{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} }\limits^{Cl{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} } } - C{H_3}\)
(c) \(C{H_3} - C{H_2} - \mathop {\mathop {C{\mkern 1mu} {\mkern 1mu} = {\mkern 1mu} {\mkern 1mu} }\limits_{|{\kern 1pt} \,\,\,\,\,{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} } }\limits_{C{H_{3\,\,\,}}} \mathop {CH - C{H_3} + \mathop {HOCl}\limits^{{\kern 1pt} - {\kern 1pt} {\kern 1pt} {\kern 1pt} + } }\limits_{\mathop {{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} }\limits_{} } \to C{H_3} - C{H_2} - \mathop {\mathop {C{\mkern 1mu} {\mkern 1mu} - }\limits_{|{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} }^{|{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} } }\limits_{{\kern 1pt} C{H_3}}^{OH} \mathop {CH}\limits^{\mathop {|{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} }\limits^{Cl{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} } } - C{H_3}\)
Addition takes place according to Markownikoff’s rule in which \(C{l^ + }\) goes to that carbon atom which is more hydrogenated.
Download our appand get started for free
Experience the future of education. Simply download our apps or reach out to us for more information. Let's shape the future of learning together!No signup needed.*
Similar Questions
- 1View Solutionનીચેનામાંથી કોનો ઉપયોગ ઇથેનથી ઇથેનને અલગ કરવા માટે થતો નથી?
- 2View Solutionએકથી વધુ પ્રકારના સંકરણ ધરાવતા કાર્બન જેમાં હોય તેવું સંયોજન..... છે.
- 3નીચેનામાંથી કયું સંયોજન ગરમ સાંદ્ર ${H_2}S{O_4}$માં પણ અદ્રાવ્ય છે?View Solution
- 4નીપજ $(P)$ શું હશે ?View Solution
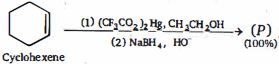
- 5View Solutionનીચેનામાંથી કયા આલ્કોહોલ એ આલ્કિન ના હાઇડ્રેશનમાંથી બનાવી શકાતા નથી?
- 6નીચેનામાંથી કયું એ સિસ અને ટ્રાન્સ - $1,4$ -ડાયમીથાઇલ સાયકલોહેકઝેન નું મિશ્રણ આપશે.જ્યારે ઉત્પ્રેરક હાઈડ્રોજીનેશન થાય છેView Solution
- 7View Solutionવ્યાપારિક ગેસોલિનમાં હાઇડ્રોકાર્બનનો ક્યો પ્રકાર વધુ ઇચ્છનીય છે ?
- 8View Solutionનીચેનામાંથી કયું સંયોજન ગુણધર્મમાં એરોમેટિક છે?
- 9નીચેની પ્રક્રિયા ક્રમમાં છેલ્લી નીપજ $(C)$ કઈ હશે?View Solution
$HC\, \equiv \,CH\,\xrightarrow[{20\% \,{H_2}S{O_4}}]{{1\% \,HgS{O_4}}}A$ $\xrightarrow[{{H_2}O}]{{C{H_3}MgX}}B\xrightarrow{{[O]}}(C)$
- 10બ્યુટા-$1,2$-ડાઇમાં રહેલા કાર્બન પરમાણુના સંકરણો........ પ્રકારના છે.View Solution
